https://doi.org/10.55788/fbf07c1d
Prof. Lorenz Räber (Bern University Hospital, Switzerland) reported the PACMAN-AMI (NCT03067844) results at the ACC 2022 Scientific Session, which were simultaneously published in JAMA [1,2]. Prof. Räber explained that the unmet need being addressed by PACMAN-AMI was that it is not well known what the impact of PCSK9 inhibition is on high-risk atherosclerotic plaque characteristics, particularly lipid content and fibrous cap thickness.
PACMAN-AMI randomised 300 patients with acute ST-elevation myocardial infarction (STEMI) (53%) or non-STEMI (47%) who had undergone successful percutaneous coronary intervention (PCI) of the culprit vessel and in whom at least 2 proximal non-infarct-related arteries had been found with non-obstructive atherosclerosis (20% to 50% diameter stenosis). Half the patients received subcutaneous 150 mg/mL alirocumab and half the patients received placebo, starting within 24 hours after PCI, and then every 2 weeks for 1 year. Only 12% of patients were on statins at baseline; LDL-cholesterol level was >125 mg/dL (3.2 mmol/L) in statin-naïve patients and >70 mg/dL (1.8 mmol/L) in statin-treated patients. The primary endpoint was the percentage change in atheroma volume. At baseline and 1 year, participants had coronary plaque characteristics in the non-infarct-related arteries assessed using intravenous ultrasound, near-infrared spectroscopy, and optical coherence tomography.
The primary endpoint at 1 year was met; a significantly greater reduction in atheroma volume was measured in the alirocumab arm compared with placebo (2.13% vs 0.92%; P<0.001; see Figure). Furthermore, while no differences were seen between trial arms in all-cause mortality and MI, a significant reduction was measured in non-infarct-related revascularisation in the alirocumab arm (8.2% vs 18.5%; P<0.001). Evidence of PCSK9 inhibition was observed in the 1 year LDL-cholesterol reduction, which averaged 50.7% in the placebo arm and 84.8% in the alirocumab arm. Key secondary endpoints also favoured the alirocumab arm: the reduction in maximum lipid core burden index within 4 mm (79.42 vs 37.60; P=0.006), as well as change in mean minimal fibrous cap thickness (62.67 μm vs 33.19 µm; P=0.001).
Figure: Primary endpoint of PACMAN-AMI [1]
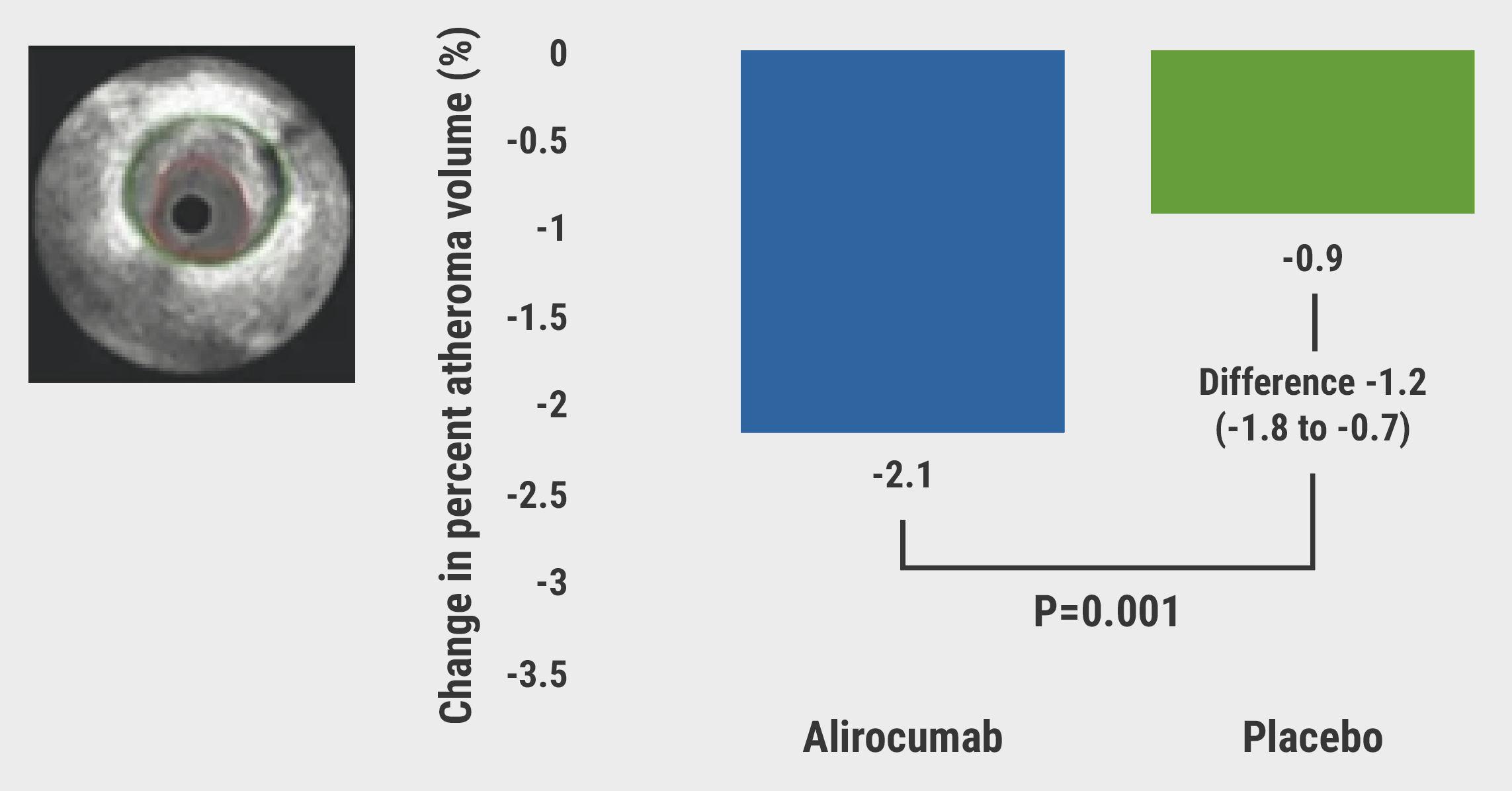
Prof. Räber noted that the “changes were greatest in patients with lower on-treatment LDL-cholesterol levels <50 mg/dL, which suggests that we should achieve very, very low LDL levels in our patients at very high risk.”
Safety results were similar in both trial arms, although there was a slightly higher occurrence of general allergic reactions in the alirocumab arm (3.4% vs 0%, respectively). Prof. Räber suggested that the findings of PACMAN-AMI could be useful when counselling patients with atherosclerosis.
- Räber L, et al. Effects Of Alirocumab On Coronary Atherosclerosis Assessed By Serial Multimodality Intracoronary Imaging In Patients With Acute Myocardial Infarction: A Double-blind, Placebo-controlled, Randomized Trial (PACMAN AMI). Abstract 405–12, ACC 2022, 2–4 April, Washington DC, USA.
- Räber L, et al. JAMA. 2022;327(18):1771-1781.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Aggressive warming during non-cardiac surgery does not improve outcomes Next Article
New VOYAGER PAD data: Should patients with both PAD and CKD get rivaroxaban? »
« Aggressive warming during non-cardiac surgery does not improve outcomes Next Article
New VOYAGER PAD data: Should patients with both PAD and CKD get rivaroxaban? »
Table of Contents: ACC 2022
Featured articles
Alirocumab significantly reduces high-risk coronary plaques
Highlighted Original Research
POISE-3: Tranexamic acid for non-cardiac surgery
Treating chronic mild hypertension during pregnancy leads to better outcomes
New VOYAGER PAD data: Should patients with both PAD and CKD get rivaroxaban?
Alirocumab significantly reduces high-risk coronary plaques
Aggressive warming during non-cardiac surgery does not improve outcomes
Heart Failure and Cardiomyopathy
DIAMOND trial: Patiromer lowers risk of severe hyperkalaemia
Replacing septal reduction therapy with mavacamten for HCM
Omecamtiv mecarbil does not impact exercise capacity of patients with HFrEF
Symptomatic obstructive hypertrophic cardiomyopathy: long-term mavacamten control
Interventional and Structural Cardiology
COMPLETE revascularisation improves angina-related QoL
Plot twist for negative FAME 3 results: early QoL benefits of PCI
1-year CLASP TR results support tricuspid regurgitation repair
Head-to-head: post-TAVR edoxaban not better than DAPT
Chocolate Touch vs Lutonix catheters
No FLAVOUR difference between FFR and IVUS for PCI guidance
Myocardial Infarction
Low-resource countries benefit from global STEMI initiative
Sodium thiosulfate ineffective at cardiac protection
ICM-guided management did not improve MACE after MI
Prevention
PACIFIC-AF: Low bleeding rates for asundexian in atrial fibrillation
RCT-IVVE trial: Do HF patients benefit from annual flu shots?
TRANSLATE-TIMI 70: Primary endpoint met but safety concerns for vupanorsen
Lipoprotein(a) slashed by 98% in APOLLO trial
Dietary intervention from your supermarket
Related Articles
June 15, 2022
Chocolate Touch vs Lutonix catheters
June 15, 2022
ICM-guided management did not improve MACE after MI
June 15, 2022
COMPLETE revascularisation improves angina-related QoL
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

