https://doi.org/10.55788/b793d016
The findings of ADAPT-TAVR (NCT03284827) were presented by Prof. Duk-Woo Park (Asan Medical Center, Korea) and simultaneously published in Circulation [1,2]. ADAPT-TAVR randomised patients who had just received a TAVR (n=229; average age 80 years; 58% women) to either receive 6 months of edoxaban or 6 months of DAPT in this head-to-head study. The primary endpoint was the incidence of subclinical leaflet thrombosis (SLT) in the 6 months after the valve replacement. Secondary endpoints were causative associations of SLT with cerebral thromboembolism in this period, as well as bleeding, and neurological or cognitive decline.
Although the results showed a trend towards a lower incidence of SLT in the edoxaban arm when compared with the DAPT arm, the numbers did not reach the predetermined significance (9.8% vs 18.4%; P=0.076; see Figure). Likewise, the secondary endpoints were also not met; upon brain MRI, the percentage of patients with new cerebral lesions on brain MRI did not significantly differ between the 2 groups (25.0% vs 20.2%, respectively). Furthermore, no differences between neurological or cognitive function were measured between the 2 arms. There were also no differences in bleeding outcomes; in a panel discussion following his presentation, Prof. Park pointed out that prior studies have shown that despite mitigated subclinical leaflet thrombosis after TAVI has been seen in patients treated with DOACs, subsequent protection from cerebral or ischaemic events has not been consistently observed and has been associated with more major bleeding [1].
Figure: ADAPT-TAVR results showed a trend toward a lower incidence of SLT in the edoxaban arm when compared with the DAPT arm in ITT population [1]
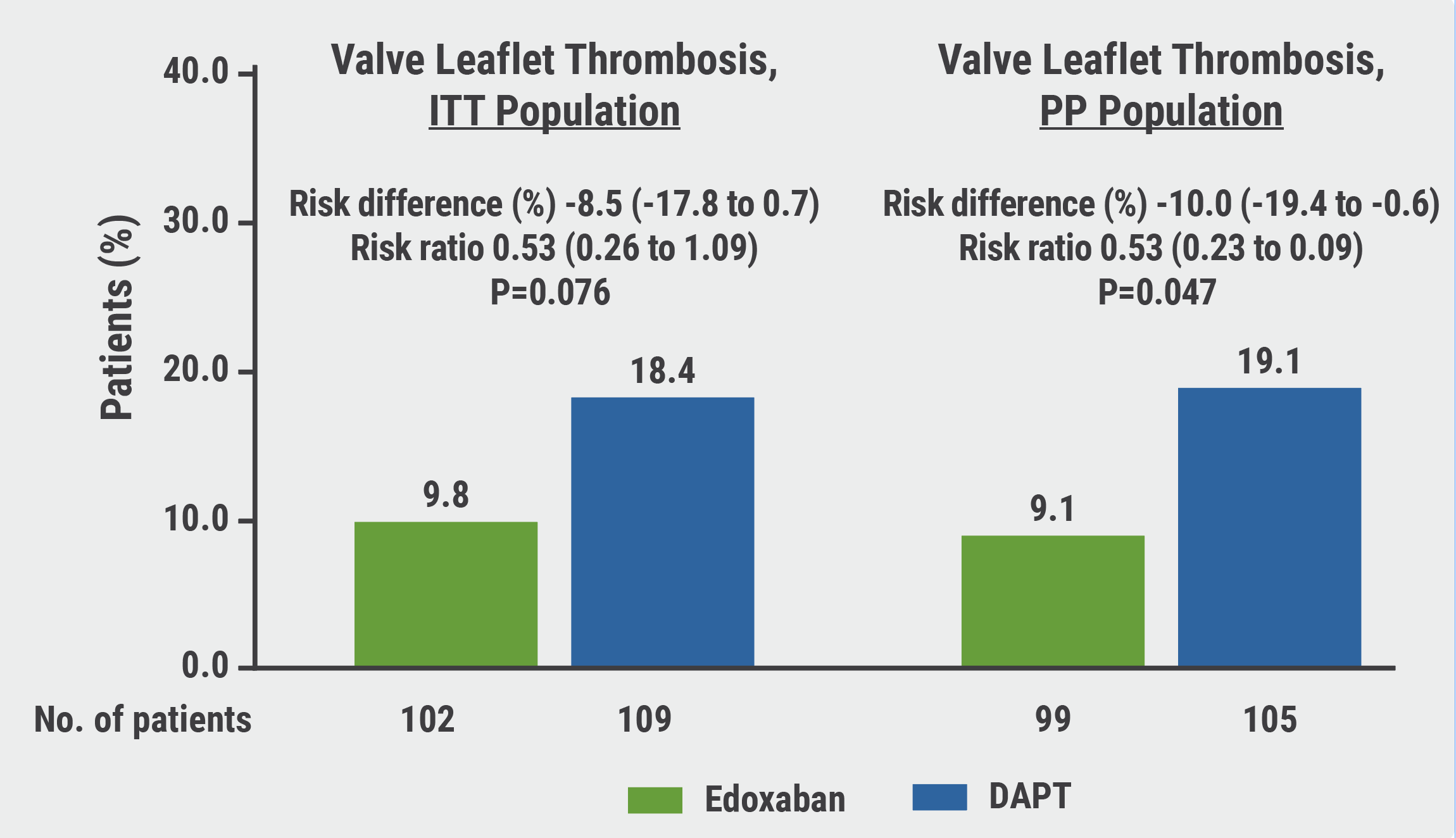
DAPT, dual antiplatelet therapy; ITT, intention-to-treat; PP, per-protocol.
Prof. Park placed the findings into context: “The key messages from this study are that SLT has not been proven to affect clinical outcomes for patients undergoing valve replacement and that its presence in patients in whom SLT causes no symptoms or complications should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve. Additionally, these findings do not support the routine use of computed tomography (CT) scans to detect SLT.”
However, Prof. Park noted that the study may have been too small to conclusively answer this question; the number of events was too small to reach significant associations with imaging scans. In addition, the 6-month follow-up period was likely too short. These considerations will be taken forward into further studies.
- Park DW, et al. Edoxaban Versus Dual Antiplatelet Therapy For Valve Thrombosis And Cerebral Thromboembolism After Transcatheter Aortic-valve Replacement: A Randomized ADAPT-TAVR Trial. Abstract 409–16, ACC 2022, 2–4 April, Washington DC, USA.
- Park DW, et al. Circulation. 2022; Apr 4. DOI:10.1161/CIRCULATIONAHA.122.059512.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Chocolate Touch vs Lutonix catheters Next Article
1-year CLASP TR results support tricuspid regurgitation repair »
« Chocolate Touch vs Lutonix catheters Next Article
1-year CLASP TR results support tricuspid regurgitation repair »
Table of Contents: ACC 2022
Featured articles
Alirocumab significantly reduces high-risk coronary plaques
Highlighted Original Research
POISE-3: Tranexamic acid for non-cardiac surgery
Treating chronic mild hypertension during pregnancy leads to better outcomes
New VOYAGER PAD data: Should patients with both PAD and CKD get rivaroxaban?
Alirocumab significantly reduces high-risk coronary plaques
Aggressive warming during non-cardiac surgery does not improve outcomes
Heart Failure and Cardiomyopathy
DIAMOND trial: Patiromer lowers risk of severe hyperkalaemia
Replacing septal reduction therapy with mavacamten for HCM
Omecamtiv mecarbil does not impact exercise capacity of patients with HFrEF
Symptomatic obstructive hypertrophic cardiomyopathy: long-term mavacamten control
Interventional and Structural Cardiology
COMPLETE revascularisation improves angina-related QoL
Plot twist for negative FAME 3 results: early QoL benefits of PCI
1-year CLASP TR results support tricuspid regurgitation repair
Head-to-head: post-TAVR edoxaban not better than DAPT
Chocolate Touch vs Lutonix catheters
No FLAVOUR difference between FFR and IVUS for PCI guidance
Myocardial Infarction
Low-resource countries benefit from global STEMI initiative
Sodium thiosulfate ineffective at cardiac protection
ICM-guided management did not improve MACE after MI
Prevention
PACIFIC-AF: Low bleeding rates for asundexian in atrial fibrillation
RCT-IVVE trial: Do HF patients benefit from annual flu shots?
TRANSLATE-TIMI 70: Primary endpoint met but safety concerns for vupanorsen
Lipoprotein(a) slashed by 98% in APOLLO trial
Dietary intervention from your supermarket
Related Articles

June 15, 2022
Letter from the Editor
June 15, 2022
Surprise outcome for SODIUM-HF
June 15, 2022
Chocolate Touch vs Lutonix catheters
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

