Prof. Josep Brugada (chairperson of the guidelines task force) reminded the audience how frequently the condition occurs [1]. “The prevalence of SVT is 2.25/1000 persons, its incidence 35/100,000 person-years. Women have a 2-fold higher risk of developing SVT than men; and in individuals aged 65 or older, the risk of developing SVT is 5 times greater than younger people.”
The 2019 guidelines provide treatment recommendations for all types of SVT. Although, drug therapies have not significantly changed since the previous guidelines were published in 2003, Prof. Brugada mentioned that more evidence has come to light over the past decade concerning the potential benefits and risks that are associated with various drugs. “This enables doctors to use these drugs even more safely. Moreover, some new anti-arrhythmic drugs have become available as well.” Although anti-arrhythmic drugs have proven to be effective in acute episodes, long-term treatment often requires other measures as these drugs show relatively low efficacy and may cause side-effects in the long run.
Catheter ablation has shown to be a useful and safe method to treat arrhythmia. Techniques and technology have evolved in such a way that this treatment modality can be offered to most patients with SVT, including pregnant women. The 2019 ESC Guidelines even hold specific recommendations for pregnant patients. “In general, all anti-arrhythmic drugs should be avoided, if possible, within the first trimester of pregnancy,” Prof. Brugada said. “Fluoroless catheter ablation should be considered in case of drug-refractory or poorly tolerated SVT, in experienced centres. Also, catheter ablation is recommended in symptomatic women with recurrent SVT who plan to become pregnant.”
The 2019 ESC Guidelines for the management of patients with supraventricular tachycardia have been published in the European Heart Journal 2019 and on the ESC website.
1. Brugada J. 2019 ESC Guidelines on Supraventricular Tachycardias. FB Number 1057. ESC Congress 2019, 31 Aug-4 Sept, Paris, France.
Posted on
Previous Article
« The Italian experience: DOAC more cost-effective than VKA Next Article
Rivaroxaban monotherapy non-inferior to combination therapy in AF and stable CAD patients »
« The Italian experience: DOAC more cost-effective than VKA Next Article
Rivaroxaban monotherapy non-inferior to combination therapy in AF and stable CAD patients »
Related Articles
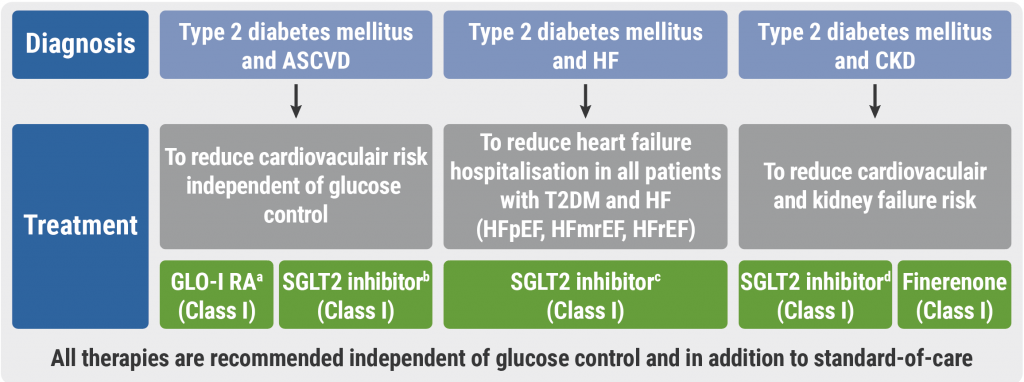
October 30, 2023
Cardiovascular disease and diabetes: new guidelines
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

