https://doi.org/10.55788/a6a40ff2
Mavacamten controlled symptomatic obstructive hypertrophic cardiomyopathy (HCM) for a median of 62 weeks and up to 84 weeks, with no additional safety concerns compared with the first 30 weeks on treatment, according to updated results from the EXPLORER-LTE cohort of the ongoing MALVA-LTE study.
The MALVA-LTE (NCT03723655) trial is an international, ongoing, 5-year extension study including patients with symptomatic obstructive HCM who completed the treatment phase of the phase 3 MAVERICK-HCM (NCT03442764) and EXPLORER-HCM (NCT03470545) trials. Mavacamten, a small molecule modulator of β-cardiac myosin, inhibits excessive myosin-actin cross-bridge formation and consequent hypercontractility, a central pathophysiological hallmark of HCM. Prof. Florian Rader (Cedars-Sinai Medical Center, CA, USA) presented the updated findings from the EXPLORER-LTE cohort of the MALVA-LTE study [1].
In the previously published EXPLORER-HCM study, participants were randomised in a 1:1 ratio to receive either a starting dose of 5 mg of mavacamten or placebo once daily for 30 weeks [2]. Prof. Radar focused on the long-term safety results of patients (n=231) taking mavacamten for a median of 62 weeks [1]. Dose adjustments were made at weeks 4, 8, and 12 based on only 2 echocardiography measures: resting left ventricular outflow tract (LVOT) gradient and left ventricular ejection fraction (LVEF).
After 48 weeks of treatment, resting LVOT gradient decreased from baseline by an average of 35.6 mmHg, which held after 84 weeks at an average 32.8 mmHg reduction. Similarly, LVOT gradient measured using the Valsalva manoeuvre reported reductions from baseline averaging 45.3 mmHg after 48 weeks and 46.4 mmHg after 84 weeks.
Likewise, LVEF dropped 7.0% ± 8.3% at week 48. A similar level of reduction persisted throughout this extension period (up to 84 weeks). Clinical benefit was first evident at week 12 as measured by improvements in New York Heart Association (NYHA) Classification and was sustained over time; by week 48, 67.5% (139/206) of patients improved by ≥1 NYHA Class from baseline, of whom 60.2% (124/206) improved by 1 NYHA Class and 7.3% (15/206) improved by 2 NYHA Classes (see Figure).
Figure: Improvements in NYHA Class with mavacamten treatment were sustained over time [1]
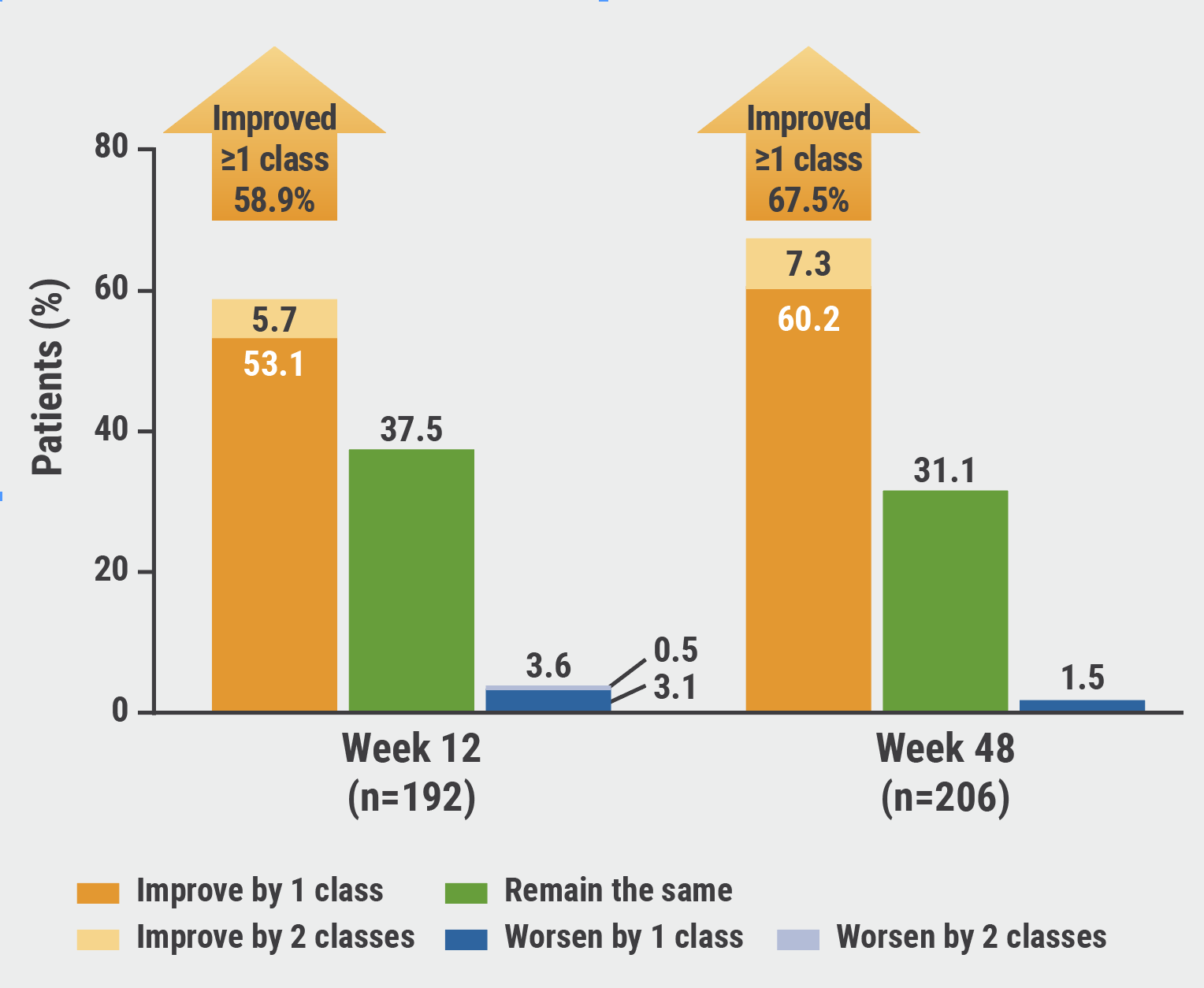
Safety results indicated that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten patients (4.3%) permanently discontinued treatment due to treatment-emergent adverse events.
- Rader F, et al. Updated Cumulative Results Of Treatment With Mavacamten From The EXPLORER-LTE Cohort Of The MAVA-LTE Study In Patients With Obstructive Hypertrophic Cardiomyopathy. Abstract 406–016, ACC 2022, 2–4 April, Washington DC, USA.
- Olivotto I, et al. Lancet. 2020;396(10253):759–769.
Copyright ©2022 Medicom Medical Publishers
Posted on
« COMPLETE revascularisation improves angina-related QoL Next Article
Omecamtiv mecarbil does not impact exercise capacity of patients with HFrEF »
Table of Contents: ACC 2022
Featured articles
Alirocumab significantly reduces high-risk coronary plaques
Highlighted Original Research
POISE-3: Tranexamic acid for non-cardiac surgery
Treating chronic mild hypertension during pregnancy leads to better outcomes
New VOYAGER PAD data: Should patients with both PAD and CKD get rivaroxaban?
Alirocumab significantly reduces high-risk coronary plaques
Aggressive warming during non-cardiac surgery does not improve outcomes
Heart Failure and Cardiomyopathy
DIAMOND trial: Patiromer lowers risk of severe hyperkalaemia
Replacing septal reduction therapy with mavacamten for HCM
Omecamtiv mecarbil does not impact exercise capacity of patients with HFrEF
Symptomatic obstructive hypertrophic cardiomyopathy: long-term mavacamten control
Interventional and Structural Cardiology
COMPLETE revascularisation improves angina-related QoL
Plot twist for negative FAME 3 results: early QoL benefits of PCI
1-year CLASP TR results support tricuspid regurgitation repair
Head-to-head: post-TAVR edoxaban not better than DAPT
Chocolate Touch vs Lutonix catheters
No FLAVOUR difference between FFR and IVUS for PCI guidance
Myocardial Infarction
Low-resource countries benefit from global STEMI initiative
Sodium thiosulfate ineffective at cardiac protection
ICM-guided management did not improve MACE after MI
Prevention
PACIFIC-AF: Low bleeding rates for asundexian in atrial fibrillation
RCT-IVVE trial: Do HF patients benefit from annual flu shots?
TRANSLATE-TIMI 70: Primary endpoint met but safety concerns for vupanorsen
Lipoprotein(a) slashed by 98% in APOLLO trial
Dietary intervention from your supermarket
Related Articles

Letter from the Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

