Dr Douglas Johns (Merck & Co., NJ, USA) mentioned that many patients with hypercholesterolaemia do not reach their LDL cholesterol treatment goals [1]. Although injectable PCSK9 inhibitors demonstrated LDL cholesterol reductions of 50–60%, these therapies are often administered as a last resort only. An oral PCSK9 inhibitor could remove the barriers associated with injectable treatments.
A first randomised, double-blind, placebo-controlled, in-human trial assessed the safety and tolerability of single doses of MK-0616 ranging from 10 mg to 300 mg in 60 male participants (aged 18–50 years). MK-0616 was generally safe and well tolerated in this population. No serious adverse events (AEs) were reported and only 1 treatment-related discontinuation was observed, a case of maculopapular rash. Drug-related AEs were mostly abdominal discomfort, diarrhoea, dyspepsia, and headache. Free PCSK9 was reduced by more than 80%, regardless of the administered dose of MK-0616. This effect lasted for approximately 24 hours. Free PCSK9 levels returned to baseline levels in 96 hours. In addition, the authors observed that a permeation enhancer (i.e. sodium caprate) improved the absorption of MK-0616 and noted a negative pre-dose food effect.
A second double-blind, placebo-controlled, phase 1 trial evaluated the LDL-cholesterol lowering capacities of MK-0616 in 40 men and women (aged 18–65 years) treated with statin therapy. Patients were randomised 3:1 to 1 of 3 dosing regimens of MK-0616 or placebo (see Figure). The 14-day result displayed no serious AEs, deaths, or discontinuations. Treatment-related AEs were similar to the reported AEs in the first trial, demonstrating a favourable safety profile of the agent. LDL cholesterol was reduced by a maximum of 65% in all experimental conditions, except for the pre-dose food condition (see Figure). This result suggests that low-dose MK-0616 plus low-dose sodium caprate can achieve a major reduction in LDL cholesterol. Larger clinical trials need to confirm the safety and efficacy of MK-0616 in a diverse population.
Figure: MK-0616 dosing and LDL-cholesterol change from baseline [1]
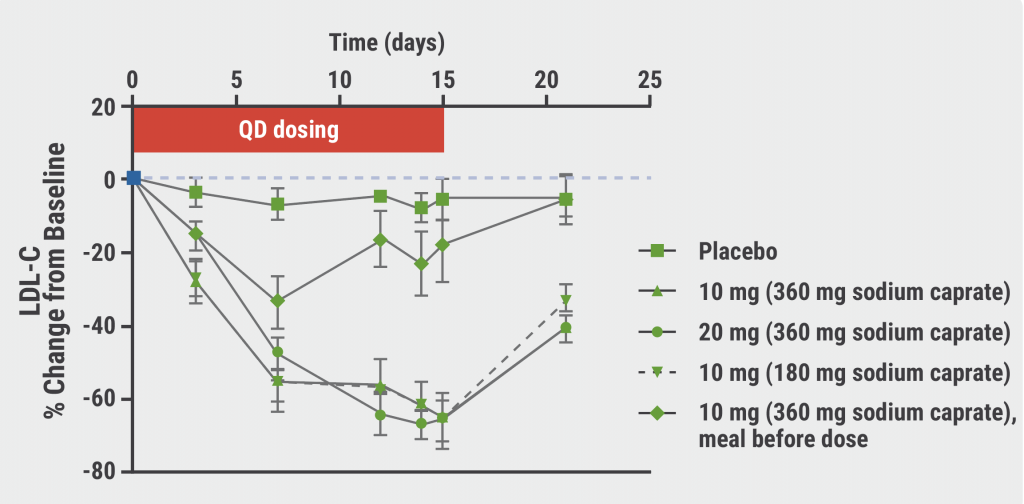
LDL-C, low-density lipoprotein cholesterol; PBO, placebo.
- Johns DG, et al. The Clinical Safety, Pharmacokinetics, and LDL-Cholesterol Lowering Efficacy of MK-0616, an Oral PCSK9 Inhibitor. LBS06, AHA Scientific Sessions 2021, 13–15 November.
Copyright ©2021 Medicom Medical Publishers
Posted on