The accuracy of minimally invasive biopsies to assess presence of pCR of the breast is currently investigated by a number of trials. Interim results of the MICRA trial (Minimal Invasive Complete Response Assessment; NTR6120), were presented at SABCS 2019 [1]. This study combines MRI and minimally invasive biopsies of the breast. The primary endpoint of the study was the false-negative rate of the biopsy procedure (which is the proportion of patients with non-pCR in the surgical specimen but with pCR in the biopsies). The main difference between the MICRA trial and other trials is that MRI was mandatory both pre- and post-neoadjuvant systemic therapy. Furthermore, the size of biopsies was 14 gauge (G), smaller than in other trials. A total of 219 breast cancer patients treated with neoadjuvant systemic therapy which resulted in a radiologic complete (rCR) or partial response (rPR, >30% decrease and <2 cm residual diameter) on MRI were enrolled, and in 167 of these biopsies were successfully obtained and analysed. Mean age of the patients was 49 years (range 24-74 years), tumour subtype was 23% hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative, 16% HR-negative/HER2-positive, 34% triple-negative, and 27% HR-positive/HER2-positive.
In total, 80 patients (59%) had a rCR and 9 patients (28%) had a rPR on MRI, resulting in a total of 89 patients (53%) with pCR in the surgical specimen (see Table). This pCR was correctly identified by post-neoadjuvant systemic therapy biopsies in all 89 cases (false-positive rate 0%). However, biopsies missed residual disease with a false-negative rate of 37% (29 of 78 patients; see Table). False-negative rate was higher in patients with rCR on MRI (45%; 26/55 patients with residual disease missed on biopsies) than in patients with rPR (13%; 3/23 patients with residual disease missed with biopsies). Subgroup analyses showed that predictive factors for false-negative biopsies were response on MRI and the definite size of residual tumour at the surgical specimen. It was thus concluded that MRI and 14G biopsies are not accurate enough in pCR detection.
Table. Pathological response and accuracy of post-neoadjuvant systemic therapy biopsy [1]
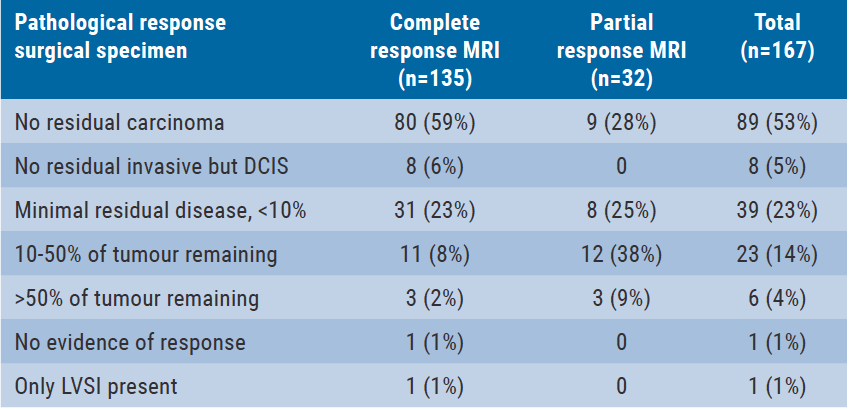

DCIS, ductal carcinoma in situ; LVSI, lymph vascular space invasion
Furthermore, researchers think it is too early to omit breast surgery after primary systemic therapy, as residual disease is missed in 2/3 patients. The consequences of missing residual disease are various: pCR is still being used as a prognostic factor and an indication for adjuvant capecitabine, although that may be overruled by other methods. Moreover, radiation is given to all these patients as well, and if missing too much residual disease would mean a higher recurrence rate, this could mean a higher rate of mastectomy. This needs to be discussed as the value of the false-negative rate is not yet known. Future perspectives should focus on improving the response prediction model by advanced MRI-PET features, advanced image analysis (deep learning), and adding biomarkers.
1. Vrancken Peeters M-JTFD, et al. GS5-06. SABCS 2019.
Posted on
Previous Article
« No higher pathologic complete response with atezolizumab added to chemotherapy Next Article
Editor Biography »
« No higher pathologic complete response with atezolizumab added to chemotherapy Next Article
Editor Biography »
Table of Contents: SABCS 2019
Featured articles
Screening, Detection, and Diagnosis
Phase 2 Trial Update
Phase 3 Trial Update
Long-Term Study Results
Triple-Negative Breast Cancer
HER2-Positive Breast Cancer
Related Articles
February 13, 2020
Clinical benefit over trastuzumab for margetuximab in HER2 breast cancer

February 13, 2020
MRI and 14G biopsies are not accurate enough in pCR detection
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

