https://doi.org/10.55788/d4e8de1e
For patients with newly diagnosed DLBCL, R-CHOP remains the standard of care. However, about 45% of the patients do not reach a curative state and the 4-year overall survival rate is only 55% [1,2]. Epcoritamab is a bispecific antibody targeting CD3 and CD20, which has demonstrated efficacy and safety in patients with relapsed or refractory DLBCL as a single agent [3]. Dr Michael Clausen (Lillebaelt Hospital, Denmark) presented the updated results from arm 1 of the phase 1b/2 EPCORETM NHL-2 trial (NCT04663347)[4]. This arm of the trial included 47 participants with untreated DLBCL and evaluated the safety and efficacy of epcoritamab (weekly in R-CHOP cycles 1-4, every 3 weeks in cycles 5–6, every 4 weeks in cycle 7 for a total of 1 year) plus R-CHOP (21-day cycles). Anti-tumour activity was the primary endpoint.
The overall response rate was 100% in the study population and therefore also 100% in double-hit/triple-hit participants (n=11). The complete metabolic response rate was 80% and the partial metabolic response rate was 20%. Of those participants who completed therapy, 95% achieved and maintained a complete response after 9 months of follow-up (see Figure).
Neutropenia (67%), anaemia (62%), and cytokine release syndrome (CRS; 59%) were the most common adverse events. There was only one grade 3 CRS event reported.
In conclusion, these promising data of epcoritamab plus R-CHOP support further evaluation of this combination regimen in patients with DLBCL.
Figure: Median duration of response and complete response for participants treated with epcoritamab plus R-CHOP [4]

Median duration of response
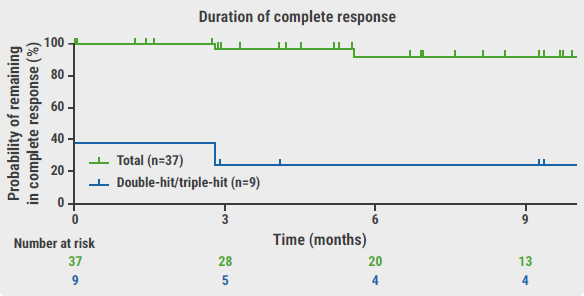
Median duration of complete response
- Sehn LH, et al. Blood. 2007;109(5):1857─1861
- Susanibar-Adaniya S, et al. Am J Hematol. 2021;96(5):617─629
- Phillips T, et al. Blood. 2022;140 (supplement 1): 8022─8023
- Clausen M, et al. High complete metabolic response rates with epcoritamab + R-chop in previously untreated patients with high-risk diffuse large B-cell lymphoma, including double/triple-hit: epcore NHL-2 update. P116, EHA 2023 Annual Congress, 8─11 June, Frankfurt, Germany.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Can we combine teclistamab and nirogacestat for the treatment of RRMM? Next Article
Zandelisib promising in relapsed/refractory indolent B-cell NHL »
« Can we combine teclistamab and nirogacestat for the treatment of RRMM? Next Article
Zandelisib promising in relapsed/refractory indolent B-cell NHL »
Table of Contents: EHA 2023
Featured articles
Multiple Myeloma
Can we combine teclistamab and nirogacestat for the treatment of RRMM?
Encouraging results for low-dose belantamab mafodotin plus nirogacestat in patients with RRMM
CARTITUDE-4: Cilta-cel meets expectations in lenalidomide-refractory MM
Lymphoma
Radiotherapy or not in patients with PMBCL after immunochemotherapy?
Durable responses for loncastuximab tesirine in relapsed/refractory DLBCL
Zandelisib promising in relapsed/refractory indolent B-cell NHL
Promising data for epcoritamab plus R-CHOP in untreated DLBCL
Non-Malignant Haematology
Investigational agent OMS906 performs well in PNH
Robust platelet responses with cevidoplenib in ITP
Leukaemia
QuANTUM-First: Updated results on quizartinib in AML with FLT3-ITD
Promising data for ziftomenib in relapsed/refractory NPM1-mutated AML
MRD-positive patients with FLT3-ITD AML may benefit from post-transplant gilteritinib
Deep responses with asciminib in CML-CP
QUIWI: First results suggest a clinical benefit of quizartinib in AML
Miscellaneous
COMMANDS trial: A paradigm shift in LR-MDS-associated anaemia
REVIVE: Rusfertide meets the primary endpoint in PV
Mapping healthy HPSC variations to diagnose haematopoietic abnormalities
High risk of death for individuals with C282Y/C282Y hereditary haemochromatosis and diabetes
Related Articles
November 5, 2020
Antisense ANGPTL3 lowers triglycerides
November 28, 2022
Diabetes not related to abnormal biomarkers of Alzheimer’s disease
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

