https://doi.org/10.55788/5b366eb0
“Our research question was whether it was possible to withdraw the treatment with anti-TNF in IBD patients in remission without an increased risk of disease relapse,” Prof. María Chaparro Sánchez (Hospital Universitario de La Princesa, Spain) explained [1]. The quadruple-blinded, placebo-controlled, phase 4 EXIT trial (NCT02994836) randomised 140 out of 159 screened patients with Crohn’s disease (44%) or ulcerative colitis (56%). The participants were in ≥6 months of clinical remission on anti-TNF and ≥3 months of concomitant immunosuppressants at stable dosages. Participants with Crohn’s disease with perianal disease were excluded. Depending on the study arm, the participants either withdrew their anti-TNF medication or continued on infliximab (87%) or adalimumab (13%) until month 12 or relapse.
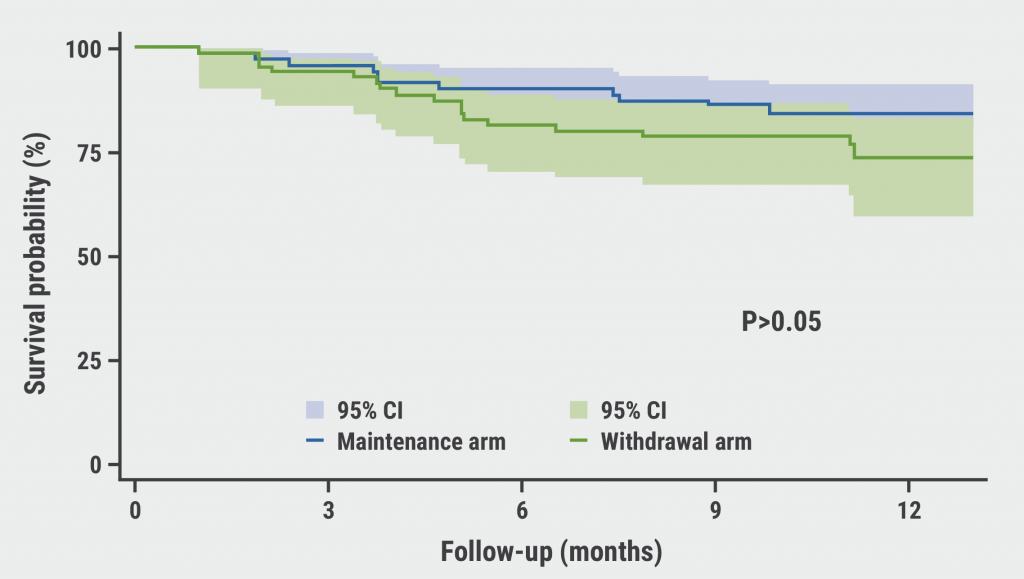
The mean age of the study population was 41 years, the median duration of anti-TNF therapy was between 2.6 and 2.9 years, and 7% had received prior biologics. Remission was sustained in 76% of the withdrawal group and 84% sustained maintenance at month 12 in the intention-to-treat population (see Figure). This difference in rates was not statistically significant, nor were the relapse percentages of 6% for maintenance and 13 % for withdrawal. “The proportion of patients with significant endoscopic lesions was also not statistically different between the groups at the last study visit,” Prof. Chaparro Sánchez indicated.
Figure: Survival curve of clinical remission at the end of follow-up [1]
After 1 year, a significant difference was observed of 33% versus 14% of patients in the withdrawal and the maintenance arm who had a faecal calprotectin >250 µg/g. In addition, a faecal calprotectin level of >250 µg/g at baseline was a significant predictor of clinical relapse. The incidence of adverse and serious adverse events was similar between the study arms.
“Anti-TNF withdrawal in selected IBD patients in clinical, endoscopic, and radiologic remission could be feasible without an increase in the risk of clinical relapse,” Prof. Chaparro Sánchez concluded. However, a long-term follow-up of these patients is warranted.
- Chaparro, M. Is the withdrawal of anti-tumour necrosis factor in inflammatory bowel disease patients in remission feasible without increasing the risk of relapse? Results from the randomised clinical trial of GETECCU (EXIT). OP37, ECCO 2023, 01–04 March, Copenhagen, Denmark.
Posted on
Previous Article
« Intensified drug therapy leads to better stricture morphology in CD Next Article
Long-term data supports the established efficacy and safety of ustekinumab in UC »
« Intensified drug therapy leads to better stricture morphology in CD Next Article
Long-term data supports the established efficacy and safety of ustekinumab in UC »
Table of Contents: ECCO 2023
Featured articles
What Is New in Biologic Therapy?
Beneficial effect of early, post-operative vedolizumab on endoscopic recurrence in CD
Long-term data supports the established efficacy and safety of ustekinumab in UC
Anti-TNF withdrawal may be a safe option in stable IBD
Intensified drug therapy leads to better stricture morphology in CD
Small Molecules in IBD: State of the Art
Continued efficacy of long-term ozanimod as UC treatment
Upadacitinib successful in the management of both CD and UC
Solid results for long-term therapy of UC with filgotinib
Paediatric IBD: What You Need To Know
Perinatal period is crucial for the risk of developing CD
Early-life antibiotic exposure: a risk factor for paediatric-onset IBD
Paediatric patients with immune-mediated inflammatory disease harbour a heightened cancer risk
Risk Factors and Complications of IBD
Checking kidney function is important during the course of IBD
Diabetes therapy with GLP-1-based drugs does not elevate the risk of IBD
Surgical Approaches: New Developments
Long-term resection potentially better than anti-TNF treatment in CD
Early, post-operative complications in CD reduced by pre-operative enteral nutrition, irrespective of biologic exposure
Pearls of the Posters
Drop in overall IBD procedures during the pandemic
Proton pump inhibitors associated with worse outcomes in CD
Poor sleep in CD linked to low levels of vitamin D
Novel AI tool assessing mucosal inflammation achieves high correlation with histopathologists
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

