https://doi.org/10.55788/821ff846
Complement activation (C5a) has been shown to play an important role in severe COVID-19 [1] and vilobelimab is a first-in-class anti-C5a monoclonal antibody that leaves the membrane attack complex (MAC) intact. Since it has been shown that C5a levels are high in patients with severe COVID-19 and related to disease severity [2], Prof. Alexander Vlaar (Amsterdam UMC, the Netherlands) and co-investigators deemed it reasonable to assess C5a inhibition in patients with severe COVID-19.
After the successful results of the PANAMO phase 2 study [3], the PANAMO phase 3 study (NCT04333420) was initiated [4]. This study randomised 368 critically-ill, intubated patients with COVID-19 1:1 to vilobelimab plus standard-of-care or to placebo plus standard-of-care. All-cause mortality after 28 days was the primary outcome.
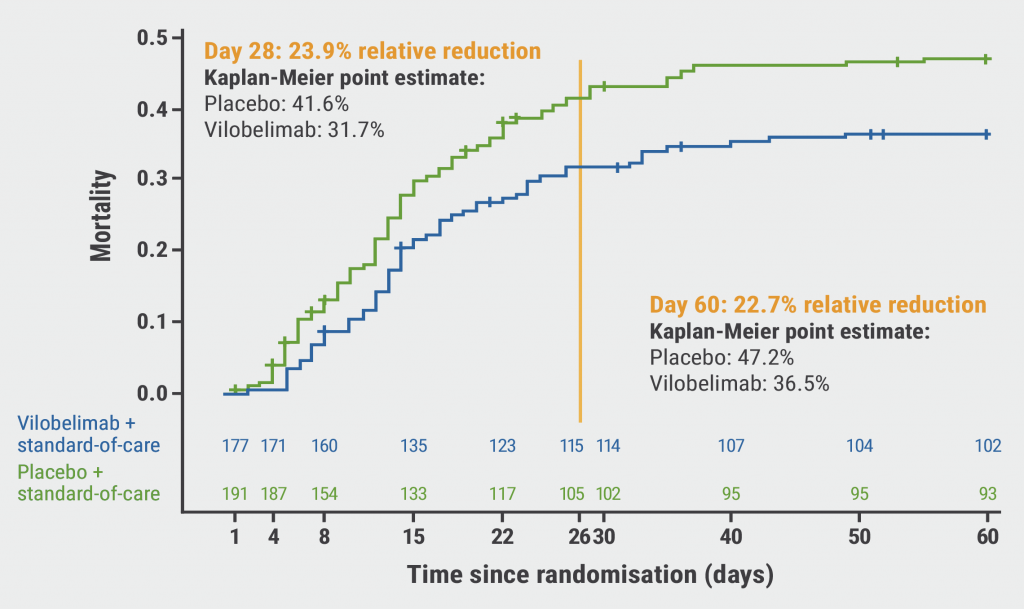
The results displayed a trend towards a reduced risk of all-cause mortality in the vilobelimab arm compared with the placebo arm (31.7% vs 41.6%; see Figure). However, this relative risk reduction of 23.9% was not statistically significant following site-stratified Cox regression (P=0.094), which was the approach recommended by the FDA. In Western-European participants receiving vilobelimab, the observed relative risk reduction for all-cause mortality was 43.0% compared with placebo. In other words, 1 additional life was saved for every 6 participants if they received vilobelimab. Furthermore, participants with more severe disease appeared to benefit more from treatment with vilobelimab than those with less severe disease.
Figure: 28-day mortality rate of vilobelimab- versus placebo-treated COVID-19 patients [4]
In terms of safety, an acceptable safety profile of vilobelimab was reported. The rate of infections and infestations was 62.9% in the intervention arm and 59.3% in the placebo arm.
“A clinically meaningful, but non-significant, benefit was observed for treatment with vilobelimab in this critically-ill population,” said Prof. Vlaar. “The developer aims to present the results to the regulatory authorities.”
- Java A, et al. JCI Insight. 2020;5(15):e140711.
- Carvelli J, et al. Nature. 2020;588:146‒150.
- Vlaar APJ, et al. Lancet Rheumatol. 2020;2(12):e764‒e773.
- Vlaar APJ, et al. Phase 3 RCT of C5a-Specific Vilobelimab in Severe COVID-19 Pneumonia. ALERT 3, RCT2881, ERS International Congress 2022, Barcelona, Spain, 4–6 September.
Posted on
Previous Article
« Awake proning not positive in COVID-19 Next Article
Hyperpolarised gas MRI ready for clinical use »
« Awake proning not positive in COVID-19 Next Article
Hyperpolarised gas MRI ready for clinical use »
Table of Contents: ERS 2022
Featured articles
Letter from the Editor
COVID-19: What Is New?
Does vilobelimab reduce mortality in severe COVID-19?
Awake proning not positive in COVID-19
Favipiravir may help patients over 60 years with COVID-19 to recover
Inhaled agent under investigation for COVID-19
Accurate voice-based COVID-19 diagnostic test in development
Novel scoring tool for post-COVID syndrome aids clinicians and researchers
COPD: Therapies and Innovations
Icenticaftor achieves results on top of triple inhalation therapy in COPD
STARR2: A new approach for treating COPD exacerbations
COPD medication not effective in symptomatic smokers with preserved spirometry
Do digital tools improve physical activity in COPD?
Hyperpolarised gas MRI ready for clinical use
All About Asthma
Tezepelumab in asthma: mucus plugging down, lung function up
Digital asthma intervention improves health and reduces costs
Digitally enhanced therapy lowers treatment burden and costs in severe asthma
Mepolizumab beneficial for patients with severe eosinophilic asthma
Progress in Paediatrics
Antibiotics cause increased risk of wheezing in severe RSV bronchiolitis
Inhaled corticosteroids useful in preterms with decreased lung function
Fish oil or vitamin D during pregnancy can prevent croup
Encouraging results of nintedanib in children with fibrosing ILD
Focus on Interventional Pulmonology
Head-to-head: lung volume reduction surgery vs endobronchial valves
Durable effect of endobronchial valves in severe emphysema
Cone beam CT-guided ENB improves detection of pulmonary nodules
Confirmatory mediastinoscopy not needed in resectable NSCLC
Sleep and Breathing Disorders
In the spotlight: Cancer trends in obstructive sleep apnoea
Impact of CPAP on cardiac endpoints in OSA
Sustained hypoxaemia predicts unprovoked VTE in OSA
CPAP therapy in the prevention of cardiovascular risk in patients with OSA
Other Remarkable Research
Excellent results for high-flow nasal cannula oxygen therapy in acute respiratory failure
Antifibrotic therapy may slow down FVC decline in RAILD
Intravenous N-acetylcysteine performs well in hospitalised patients
Men and women respond differently to diesel exhaust
New trends in cystic lung diseases
Related Articles
November 28, 2019
One blood eosinophil count is sufficient to guide ICS therapy
January 17, 2022
Secondhand vaping linked with respiratory symptoms in young adults
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

