https://doi.org/10.55788/32072916
“We’ve known for a long time now that HD ICS is associated with significant adverse effects,” Prof. David J. Jackson (King's College London, UK) stated. He pointed out that the Global Initiative for Asthma (GINA) recommends reducing HD ICS for asthma patients who are on biologics. “Unusually for GINA, this recommendation is not based on any evidence whatsoever, because the SHAMAL trial (NCT04159519) is the first study that has been done prospectively, to assess whether this is safe and possible,” Prof. Jackson further underlined.
SHAMAL is an open-label, active-controlled, phase 4 open study that investigated the possibility of a successful HD ICS reduction in patients with severe eosinophilic asthma, well-controlled with add-on benralizumab [1]. In total, 168 adults were randomised 1:3 to either continuation of their benralizumab dose at 30 mg every 8 weeks plus HD ICS/F as before or tapering of HD ICS/F from medium to low dose and further down to maintenance of anti-inflammatory reliever (AIR). The primary endpoint was defined as the proportion of patients reducing HD ICS/F to medium or low dose or the as-needed regimen only at the end of the initial period over 32 weeks.
The overall baseline parameters of the participants showed a mean age of 57.7 years, 53% were women, a mean fractional exhaled nitric oxide (FeNO) of 27.0 ppb, a mean Asthma Control Questionnaire (ACQ)-5 score of 0.53, and 2.9 exacerbations within the last year before starting benralizumab.
At week 32, nearly all participants (92%) were able to successfully reduce HD ICS/F, with 15% tapering to medium dose, 17% to low dose, and 61% to AIR only. This corresponds to an overall 73% lesser mean daily dose of HD ICS/F in the tapering arm (see Figure). Furthermore, 95.8% of the participants were able to maintain their reduced doses to week 48. Among the participants who reduced their medication to AIR, changes in lung function measured by forced expiratory volume in 1 second (FEV1) and FeNO revealed a significant correlation, indicating a decline in FEV1 and an increase in FeNO from baseline (P=0.0003 at week 32 and P=0.0241 at week 48). “Interestingly, this was not seen with exacerbations,” Prof. Jackson informed, revealing that almost all the participants remained completely exacerbation-free, as only 8% had 1 exacerbation over the study year. These results were also mirrored by the absence of a clinically meaningful decrease in symptom control in the ACQ-5.
“Most patients with severe eosinophilic asthma adequately controlled on benralizumab and background therapy can reduce ICS/formoterol dose from high dose, whilst maintaining asthma control,” Prof. Jackson concluded.
Figure: Development of change in mean total ICS per day over time [1]
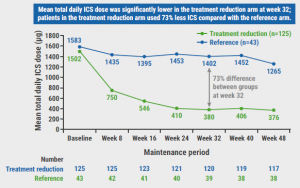
- Jackson D. SHAMAL: reduction of maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab: a randomised phase 4 study. Abstract 798, ERS International Congress 2023, 9–13 September, Milan, Italy.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Tezepelumab therapy: hints toward a disease-modifying effect? Next Article
Mechanism of autophagy in a newborn responsible for deleterious effect of air pollutants »
« Tezepelumab therapy: hints toward a disease-modifying effect? Next Article
Mechanism of autophagy in a newborn responsible for deleterious effect of air pollutants »
Table of Contents: ERS 2023
Featured articles
Letter from the Editor
Best of the Posters
sRAGE: A novel potential biomarker to assess the risk of acute respiratory events
Most severe asthma patients are candidates for biologic therapy on a global scale
Aspergillus infections: resistance to azole treatment increased in the presence of diesel particles
Asthma in 2023
Tapering from high-dose inhaled corticosteroids possible in most asthma patients treated with benralizumab
Tezepelumab therapy: hints toward a disease-modifying effect?
Digital inhaler programme improves asthma control also in the long term, but not long-term adherence
Respiratory health in children
Large infant study demonstrates the importance of a mature microbiome
Healthy maternal lifestyle during pregnancy reduces wheezing and rhinitis in infants
Mechanism of autophagy in a newborn responsible for deleterious effect of air pollutants
COPD: New Developments
Gabapentinoids increase risk of exacerbations in COPD
Future treatment of fatigue in COPD: 4 possible targets identified
Pulmonary Consequences of Long COVID
Women at higher risk of functional respiratory complaints following a COVID-19 infection
Elevated myeloid inflammation and complement activation present in various phenotypes of long COVID
Pulmonary Arterial Hypertension (PAH): Novel Developments
Encouraging long-term outcomes observed in the treatment of PAH with sotatercept
Chronic thromboembolic pulmonary hypertension: surgery entails encouraging long-term results
Women with pulmonary hypertension have better survival chances than men
Rare Diseases in 2023
Primary ciliary dyskinesia: Idrevloride shows promising results in phase 2 trial
Promising new agent as treatment for pulmonary fibrosis
Novel immunomodulator offers hope to reduce steroid dependency in sarcoidosis
Other Research of Interest
Tacrolimus versus cyclosporin: Less lung graft dysfunction
CPAP effective in reducing cardiovascular mortality in a practice study
Gefapixant curbs chronic cough independent of its duration
Related Articles
December 18, 2020
COVID-19 far more deadly than seasonal flu, large study confirms
November 26, 2020
Many with vaping lung injury may have lingering symptoms
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

