https://doi.org/10.55788/dd13faa3
PACE
The phase 3 PACE trial (NCT03147287) was designed to explore the activity of continuation of CDK4/6 inhibitor beyond progression, with a change of endocrine therapy to fulvestrant, and to explore the addition of PD-L1 inhibition. Dr Erica Mayer (Dana-Farber Cancer Institute, MA, USA) presented the results [1].
PACE enrolled 220 patients with ER-positive/HER2-negative metastatic breast cancer who progressed on CDK4/6 inhibition plus endocrine therapy. Participants were randomised 1:2:1 to receive fulvestrant alone, fulvestrant/palbociclib, or fulvestrant/palbociclib/avelumab (triple therapy). The primary objective was to study the superiority of fulvestrant/palbociclib over fulvestrant alone.
Fulvestrant with palbociclib beyond progression did not improve PFS compared with fulvestrant alone: median PFS was 4.8 months for fulvestrant alone versus 4.6 months for fulvestrant/palbociclib (HR 1.11; P=0.62). However, triple therapy did numerically increase the median PFS to 8.1 months (HR vs fulvestrant: 0.75; P=0.23). The PFS rates at 12 months were 17.5%, 13.1%, and 35.6% for fulvestrant alone, fulvestrant/palbociclib, and triple therapy, respectively. In addition, an exploratory analysis of baseline mutation status showed favourable outcomes of fulvestrant/palbociclib versus fulvestrant alone in patients with a mutation in PIK3CA, ERR1, or Rb1.
“Among patients with HR-positive/HER2-negative metastatic breast cancer, combining palbociclib with fulvestrant beyond progression on prior CDK4/6 inhibition did not significantly improve PFS compared with using fulvestrant alone. However, the observed longer PFS when a PD-L1 inhibitor was added to fulvestrant/palbociclib is an intriguing signal in this population and deserves further study,” Dr Mayer concluded.
EMERALD & SERENA-2
Both the EMERALD and SERENA-2 trials explored the efficacy of a next-generation, selective oestrogen-receptor degrader (SERD), elacestrant and camizestrant, respectively. Elacestrant has demonstrated a statistically significant improvement in PFS and a manageable safety profile compared with a single-agent endocrine therapy in the EMERALD trial (NCT03778931) [2].
EMERALD enrolled 477 patients with advanced/metastatic ER-positive/HER2-negative breast cancer who progressed on CDK4/6 inhibition. Participants were 1:1 randomised to receive elacestrant or standard-of-care (SOC) endocrine therapy (fulvestrant, anastrozole, letrozole, or exemestane). Prof. Virginia Kaklamani (University of Texas Health Sciences Center, TX, USA) presented the results from a post-hoc analysis of EMERALD on the impact of duration of prior CDK4/6 inhibition on PFS [3].
Longer duration of CDK4/6 inhibition proved to be associated with higher PFS rates, both in the elacestrant arm and the SOC arm. However, this was more pronounced in the elacestrant arm resulting in a larger absolute benefit of elacestrant with longer duration of CDK4/6 inhibition. The absolute benefit of elacestrant over SOC in the PFS rate at 18 months was 13.03% in patients with at least 6 month of CDK4/6 inhibition and 16.92% in patients with at least 18 months of CDK4/6 inhibition. The benefit of elacestrant over SOC was even more pronounced in patients with ESR1-mutated tumours. The absolute benefit of elacestrant over SOC in the PFS rate at 18 months was 20.70% in patients with at least 6 month of CDK4/6 inhibition and 30.68% in patients with at least 18 months of CDK4/6 inhibition.
Likewise, in the phase 2 SERENA-2 trial (NCT04214288), treatment with camizestrant after progression on CDK4/6 inhibition showed to be superior to treatment with fulvestrant. Again, the benefit of camizestrant over fulvestrant was more pronounced in patients with ESR1-mutated tumours [4].
CAPItello-291
Targeting the PI3K-AKT-mTOR intracellular pathway could be an alternative treatment option beyond CDK4/6 inhibition. The phase 3 CAPItello-291 trial (NCT04305496) evaluated the efficacy of capivasertib, an AKT inhibitor. A total of 708 patients with advanced HR2-positive/HER2-negative breast cancer who progressed after endocrine therapy (about 70% had CDK4/6 inhibition) were 1:1 randomised to receive capivasertib plus fulvestrant or placebo plus fulvestrant. The median PFS was significantly improved in the capivasertib arm compared with the placebo arm (7.2 vs 3.6 months; HR 0.60; P<0.001; see Figure) [5]. The overall survival data are not yet mature.
Figure: Investigator-assessed PFS for capivasertib versus placebo in the overall population [5]
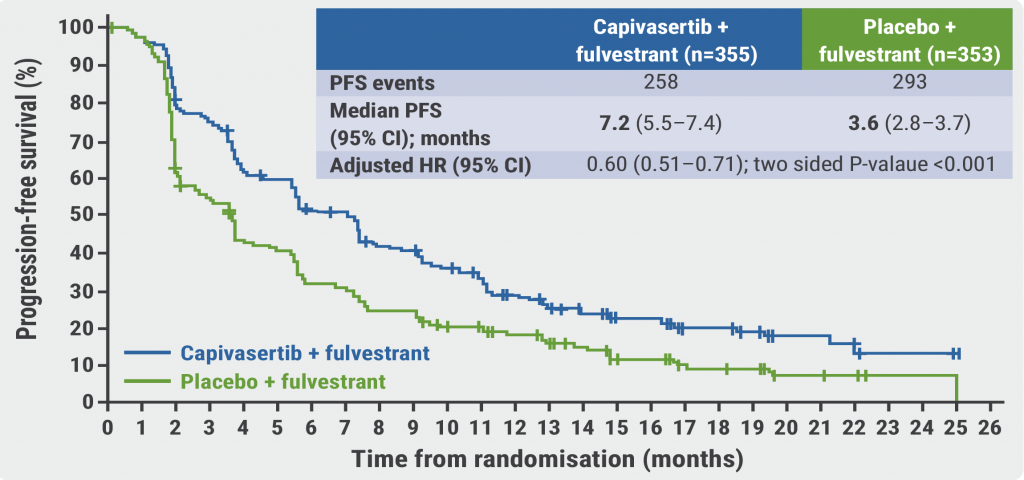
PFS, progression-free survival.
VERITAC
Finally, result from the phase 1/2 VERITAC trial (NCT04072952) showed clinical activity of ARV-471, a selective, orally-administered PROTAC protein degrader that targets wildtype and mutant oestrogen receptor in patients with advanced HR-positive/HER2-negative, advanced/metastatic breast cancer who progressed after treatment with 1 or more lines of CDK4/6 inhibition [6]. The phase 3 VERITAC-2 trial (NCT05654623) will compare the efficacy of ARV-471 with fulvestrant beyond CDK4/6 inhibition.
- Mayer EL, et al. PACE: palbociclib after CDK and endocrine therapy. A randomized phase II study of fulvestrant +/- palbociclib after progression on CDK4/6 inhibitor for HR+/HER2- metastatic breast cancer. Abstract GS3-06, SABCS 2022, 6–10 December, San Antonio, TX, USA.
- Bidard FC, et al. J Clin Oncol. 2022; 40:3246–3256.
- Bardia A, et al. EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting. Abstract GS3-01, SABCS 2022, 6–10 December, San Antonio, TX, USA.
- Oliviera M, et al. Camizestrant, a next-generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose phase 2 SERENA-2 trial. Abstract GS3-02, SABCS 2022, 6–10 December, San Antonio, TX, USA.
- Turner NC, et al. Capivasertib and fulvestrant for patients with aromatase inhibitor-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the Phase III CAPItello-291 trial. Abstract GS3-04, SABCS 2022, 6–10 December, San Antonio, TX, USA.
- Hurvitz SA, et al. ARV-471, a PROTAC® estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: phase 2 expansion (VERITAC) of a phase 1/2 study. Abstract GS3-03, SABCS 2022, 6–10 December, San Antonio, TX, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Trastuzumab deruxtecan effective in both second-line and neoadjuvant setting Next Article
First-line ribociclib plus endocrine therapy outperforms combination chemotherapy »
« Trastuzumab deruxtecan effective in both second-line and neoadjuvant setting Next Article
First-line ribociclib plus endocrine therapy outperforms combination chemotherapy »
Related Articles
September 23, 2021
Residual breast cancer burden after neoadjuvant chemo predicts survival
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

