https://doi.org/10.55788/3417c453
In patients with advanced breast cancer with aggressive disease features, like rapidly progressing or highly symptomatic disease, including life-threatening visceral crisis, combination chemotherapy is the standard-of-care [1]. The phase 2 RIGHT Choice trial (NCT03839823) compared the efficacy and safety of ribociclib plus endocrine therapy with combination chemotherapy in patients with aggressive, HR-positive/HER2-negative advanced breast cancer. Dr Yen-Shen Lu (National Taiwan University Hospital, Taiwan) presented primary results [2].
The trial randomised 222 patients with no prior systemic treatment for advanced breast cancer 1:1 to receive ribociclib plus letrozole or anastrozole or a physicians’ choice combination chemotherapy (docetaxel/capecitabine, paclitaxel/gemcitabine, or capecitabine/vinorelbine). The primary endpoint was PFS.
After a median duration of 24 months, 45.5% of patients in the ribociclib arm and 23.6% of patients in the chemotherapy arm were still on treatment. The median PFS was 24.0 months for patients treated with ribociclib plus endocrine therapy versus 12.3 months for patients treated with combination chemotherapy (HR 0.54; P=0.0007; see Figure). PFS benefit of ribociclib plus endocrine therapy was observed across most subgroups of patients. The median time to treatment failure was longer in the ribociclib than the chemotherapy arm (18.6 vs 8.5 months; HR 0.45). Time to onset of response, overall response rate, and clinical benefit rate were similar in both treatment arms.
Figure: Ribociclib plus endocrine therapy has greater PFS benefit than combination chemotherapy [2]
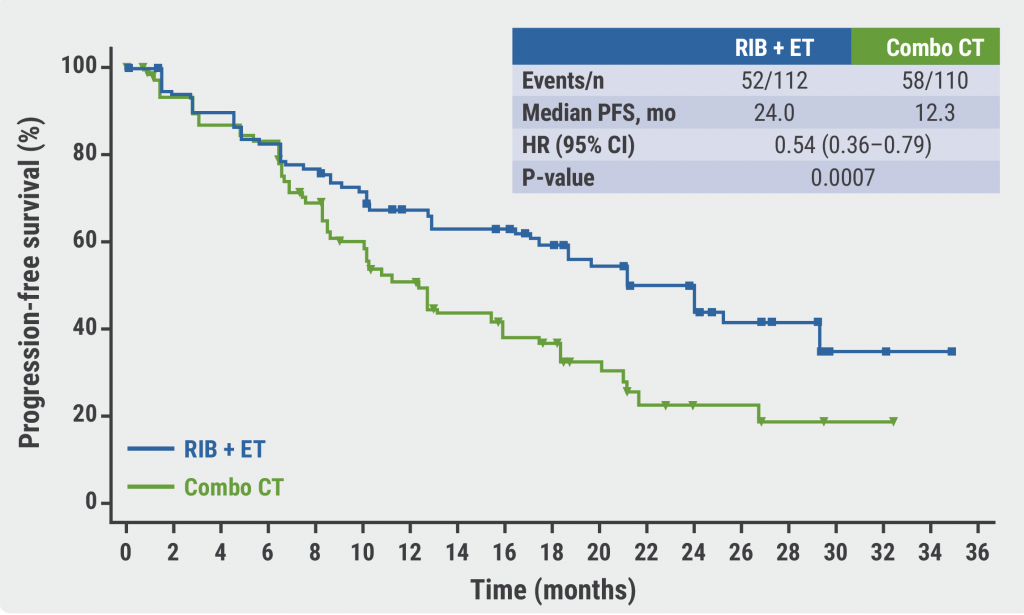
RIB + ET, ribociclib plus endocrine therapy; Combo CT, combination chemotherapy; PFS, progression-free survival; mo, months.
Based on these first results, Dr Lu concluded that “first-line ribociclib plus endocrine therapy offers an efficacious and clinically meaningful treatment option for patients with aggressive, HR-positive/HER2-negative advanced breast cancer.”
- Cardoso F, et al. Ann Oncol. 2020;31:1623–1649.
- Lu Y-S, et al. Primary results from the randomized phase II RIGHT Choice trial of premenopausal patients with aggressive HR+/HER2− advanced breast cancer treated with ribociclib + endocrine therapy vs physician’s choice combination chemotherapy. Abstract GS1-10, SABCS 2022, 6–10 December, San Antonio, TX, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Treatment options beyond CDK4/6 inhibition Next Article
Benefit of adjuvant abemaciclib continues to deepen at longer follow-up »
« Treatment options beyond CDK4/6 inhibition Next Article
Benefit of adjuvant abemaciclib continues to deepen at longer follow-up »
Table of Contents: SABCS 2022
Featured articles
Miscellaneous
Racial disparity in the tumour microenvironment
Chemo-endocrine therapy worse for cognition than endocrine therapy alone
Early-Stage Breast Cancer
Anti-PD-1/anti-LAG-3 combination highly effective in HER2-negative breast cancer
MammaPrint test predictive for benefit of extended endocrine therapy
HR-positive/HER2-positive Breast Cancer: Trastuzumab-Deruxtecan
Trastuzumab deruxtecan effective in both second-line and neoadjuvant setting
HR-positive/HER2-negative Advanced Metastatic Breast Cancer
Benefit of adjuvant abemaciclib continues to deepen at longer follow-up
First-line ribociclib plus endocrine therapy outperforms combination chemotherapy
Treatment options beyond CDK4/6 inhibition
Triple-Negative Breast Cancer
Baseline CTC count can guide first-line treatment in HR-positive/HER-negative metastatic breast cancer
ZNF689 deficiency promotes intratumour heterogeneity and resistance to immune checkpoint blockade in TNBC
Oestradiol represses anti-tumoural immune response to promote progression of brain metastases
Basic and Translational Research
Resistance to CDK4/6 inhibitors is likely due to expansion of pre-existing resistant clones
Germline pathogenic variants for breast cancer also increase contralateral breast cancer risk
Low-dose tamoxifen still prevents recurrence from non-invasive breast cancer
Endocrine interruption to pursue pregnancy does not impact short-term disease in breast cancer
Related Articles

February 28, 2022
One in seven breast cancers “overdiagnosed” in women 50-74
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

