https://doi.org/10.55788/159ca628
PD-L1 scoring based on the combined positive score (CPS) has shown predictive value for checkpoint inhibitors [1,2]. Although CPS is well-established, it remains a labour-intensive process with heterogeneous results between centres.
The TAP scoring system [3], which evaluates both immune and tumour cells to generate a score for PD-L1 tumour area, was validated for advanced gastric or gastro-oesophageal junction (G/GEJ) cancer in the RATIONALE-305 study (NCT03777657) and for advanced or metastatic oesophageal squamous cell carcinoma (ESCC) in RATIONALE-306 (NCT03783442) [4,5].
In the phase 3 RATIONALE-305 trial (n=1,657), tislelizumab plus chemotherapy demonstrated a significant overall survival (OS) benefit versus placebo plus chemotherapy as first-line therapy, in all randomised participants (HR 0.80; 95% CI 0.70–0.92; P=0.001) and participants with PD-L1 TAP scores ≥5% (HR 0.71; 95% CI 0.58–0.86) [6].
The phase 3 RATIONALE-306 trial recently demonstrated a superior OS with first-line tislelizumab plus chemotherapy compared with placebo plus chemotherapy, in all participants (HR 0.66; 95% CI 0.54–0.80) and in participants with PD-L1 TAP scores ≥10% (HR 0.62; 95% CI 0.44–0.86) [7].
In both trial cohorts, researchers directly compared the prognostic value of CPS versus TAP score [4,5]. The results showed that PD-L1 status was comparable across arms by TAP score or CPS under different thresholds. Both TAP and CPS scores similarly predicted OS and PFS in patients with PD-L1 1%, 5%, and 10% cut-off thresholds (see Figure). In addition, a good correlation was observed between TAP score and CPS based on the interclass correlation coefficient (ICC 0.81 and 0.85 in respective trials). TAP and CPS scores also showed substantial concordance in terms of Cohen’s Kappa and overall percent agreement (OPA) at matched thresholds for each score.
Figure: OS improvement for tislelizumab plus chemotherapy versus placebo plus chemotherapy across PD-L1 subgroups by TAP score and CPS in RATIONALE-306 [5]
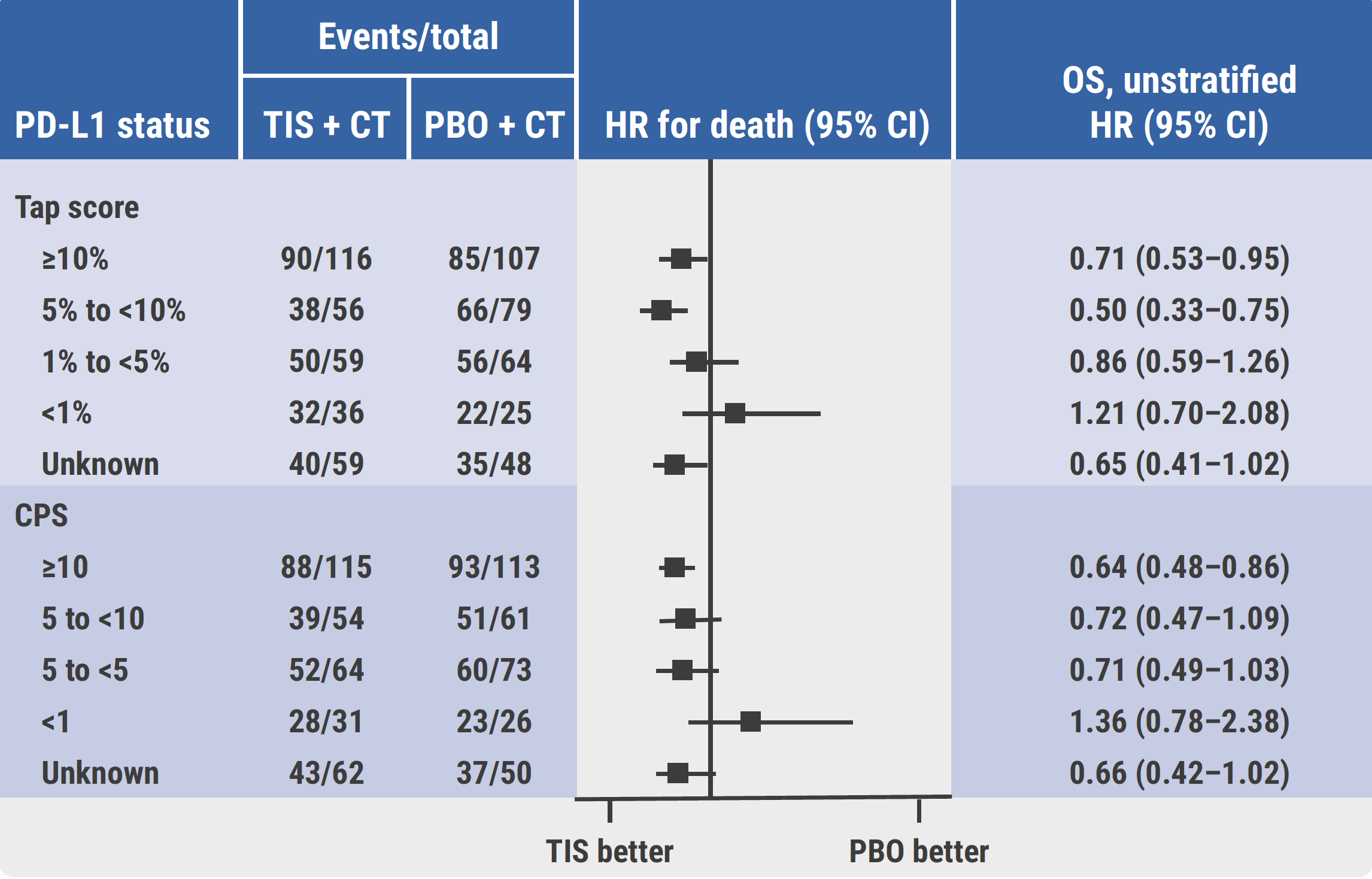
CPS, combined positive score; CT, chemotherapy; HR, hazard ratio; OS, overall survival; PBO, placebo; TAP, tumour area positivity; TIS, tislelizumab.
The conclusions from these analyses are that both TAP and CPS scores are viable for PD-L1 expression measurement in patients with G/GEJ cancer and ESCC. TAP score and CPS at matched thresholds exhibited substantial concordance among patients. Tislelizumab plus chemotherapy improved OS and PFS of patients within prespecified PD-L1 subgroups by TAP score, and demonstrated comparable OS and PFS results in PD-L1 subgroups by matched CPS.
- Shitara K, et al. Nature. 2022;603(7903):942-948.
- Rha SY, et al. Lancet Oncol. 2023;24(11):1181-1195.
- Liu C, et al. Diagn Pathol. 2023;18:48.
- Moehler M, et al. Tislelizumab (TIS) plus chemotherapy (CT) vs placebo (PBO) plus CT in HER2-negative advanced or metastatic gastric or gastro-esophageal junction adenocarcinoma (GC/GEJC): PD-L1 biomarker analysis from RATIONALE-305. Abstract 397MO, ESMO Gastrointestinal Cancers Congress 2024, 26–29 June, Munich, Germany.
- Raymond E, et al. Tislelizumab (TIS) + chemotherapy (CT) vs placebo (PBO) + CT in advanced or metastatic esophageal squamous cell carcinoma (ESCC): PD-L1 biomarker analysis from RATIONALE-306. Abstract 395MO, ESMO Gastrointestinal Cancers Congress 2024, 26–29 June, Munich, Germany.
- Qiu MZ, et al. BMJ. 2024;385:e078876.
- Xu J, et al. Lancet Oncol. 2023;24(5):483-495.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« CheckMate 8HW: Nivolumab/ipilimumab in MSI-H/dMMR mCRC Next Article
Meta-analysis of triplet therapy in BRAFV600E-mutated mCRC »
« CheckMate 8HW: Nivolumab/ipilimumab in MSI-H/dMMR mCRC Next Article
Meta-analysis of triplet therapy in BRAFV600E-mutated mCRC »
Table of Contents: ESMO GI 2024
Featured articles
Gastric and Oesophageal Cancer
OS benefit in ARMANI, but is it worth it?
SPOTLIGHT on new targets in immunotherapy: claudin 18.2
Encouraging efficacy of anti-claudin 18.2 ADC in G/GEJ cancer
New analyses validate TAP and CPS scores for PD-L1 expression
KEYNOTE-585: negative trial, but long-term benefit in PD-L1-high/MSI subgroups?
Cancers of the Pancreas, Small Bowel, and Hepatobiliary Tract
AI facilitates early detection of hepatocellular carcinoma
177Lu-DOTATATE significantly extends PFS in patients with GEP-NETs, regardless of grade or origin
Durvalumab plus chemotherapy enhances 3-year survival in advanced biliary tract cancer
Promising first results of mitazalimab in metastatic pancreatic ductal adenocarcinoma
Cancers of the Colon, Rectum, and Anus
Post-operative MRD status more prognostic than TNM stage
CAPRI 2 GOIM trial navigates biomarker-driven therapy
Meta-analysis of triplet therapy in BRAFV600E-mutated mCRC
CheckMate 8HW: Nivolumab/ipilimumab in MSI-H/dMMR mCRC
Sequence effect for third-line treatment of mCRC
REGINA meets stage 1 endpoint in rectal cancer and moves to stage 2 with reduced dose regorafenib
High efficacy of pembrolizumab combined with standard therapy in patients with MSS/pMMR mCRC and high immune infiltrate
Prognostic value of ctDNA in stage III colon cancer
Neoadjuvant combined immunotherapy also effective in MSS/pMRR CRC
General GI Cancer
Peri- or post-operative chemotherapy benefits patients with resectable CRCLM
MINOTAUR: Promising phase 1 data for lunresertib plus FOLFIRI
Related Articles

March 21, 2022
Dostarlimab may offer an alternative for dMMR rectal cancer
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

