https://doi.org/10.55788/c16e9006
Sparsentan is a dual endothelin angiotensin receptor antagonist, a novel investigational molecule that selectively targets the endothelin A receptor and the angiotensin II subtype 1 receptor. In forms of rare chronic kidney disease, blockade of both endothelin type A and angiotensin II type 1 pathways has shown to be able to reduce proteinuria, protect podocytes, and prevent glomerulosclerosis and mesangial cell proliferation. The 8-week, double-blind period of the phase 2 DUET trial (NCT01613118) in patients with FSGS (excluding secondary FSGS), sparsentan (200, 400, and 800 mg/day) resulted in greater proteinuria reduction versus irbesartan 300 mg/day. Dr Tarak Srivastava (Children’s Mercy Hospital, USA) presented the 240-week analysis of the DUET open-label extension (OLE) study which assessed the on-treatment long-term efficacy and safety of sparsentan. A total of 108 patients who received ≥1 sparsentan dose were examined from the first sparsentan dose (double-blind or OLE) for 240 weeks (4.6 years). Outcomes examined in the 240-week analysis included proteinuria, estimated glomerular filtration rate, blood pressure, and most common TEAES. According to Dr Srivastava, 43% of patients experienced ≥1 complete remission of proteinuria at any time. “The chronic slope estimate all on-treatment data is −4.16 (95% CI −5.8 to −2.5) mL/min/1.73m2. There was also a sustained reduction in blood pressure of between 8 and 10 units in systolic blood pressure and 6 and 8 units in diastolic pressure.” The most common TEAEs are outlined below (see Table).
Table: Most common treatment-related adverse events
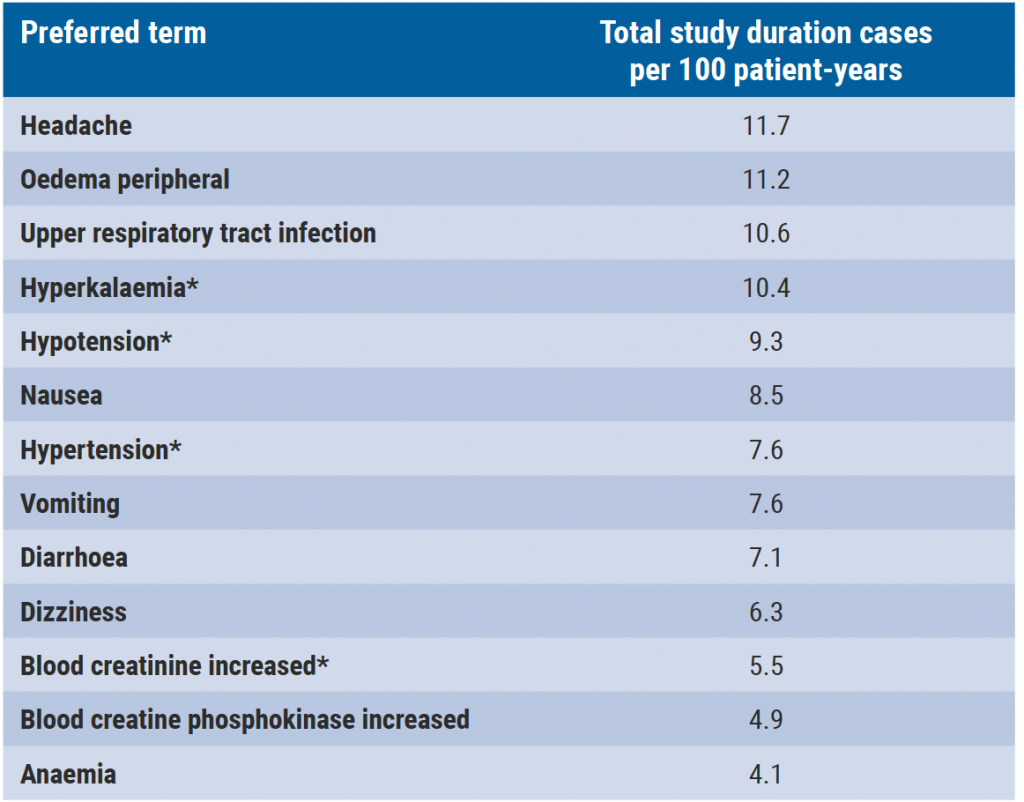
Dr Srivastava mentioned that the ongoing phase 3 DUPLEX study (NCT03493685) is evaluating the long-term antiproteinuric efficacy and safety of sparsentan in FSGS over a double-blind period of 112 weeks followed by an OLE of 156 weeks.
- Srivastava T, et al. Long-Term Efficacy and Safety of Sparsentan in FSGS: 240-Week Analysis of the DUET Open-Label Extension (OLE). FR-OR57, ASN Kidney Week 2022, 3–6 Nov.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Encaleret normalises mineral homeostasis in patients with ADH1 Next Article
Cemdisiran shows promise in IgA nephropathy »
« Encaleret normalises mineral homeostasis in patients with ADH1 Next Article
Cemdisiran shows promise in IgA nephropathy »
Table of Contents: ASN 2022
Featured articles
Chronic Kidney Disease
VALOR-CKD trial did not show any benefits for veverimer
EMPA-KIDNEY: empagliflozin slashes kidney disease progression or CV death
Combining UACR and GFR improves prediction of drug effect in CKD phase 2 trials
Dapagliflozin reduces number of hospitalisations in patients with CKD
Novel MSC therapy appears safe and effective in preventing decline in eGFR
Dapagliflozin improves anaemia in patients with CKD with or without T2D
Kidney Transplantation and Dialysis
Balanced crystalloid solution better for deceased donor kidney transplantations
Modified donor blood cells seem a promising option in kidney transplant recipients
Cooler dialysate does not offer any clinical benefits
Antiviral effect of MAU868 against BK virus prompts further research
General Nephrology
Medication-targeted alerts for the risk of AKI
Coaching with a DASH diet improves albuminuria
Cemdisiran shows promise in IgA nephropathy
Long-term nephroprotective effects of sparsentan in FSGS
Encaleret normalises mineral homeostasis in patients with ADH1
Adding voclosporin to MMF and steroids results in long-term higher CRR in severe lupus nephritis
Selonsertib poses risk of AKI in patients with DKD
Significantly higher risk of overcorrection in hyponatraemic patients with standard bolus infusion
Lowering blood pressure intervention favourable for CV outcomes
Related Articles
June 20, 2024
Zigakibart slows down eGFR decline in IgA nephropathy

January 4, 2024
Interview: Medicare’s first drug price negotiations: concerning?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

