https://doi.org/10.55788/dcf900a3
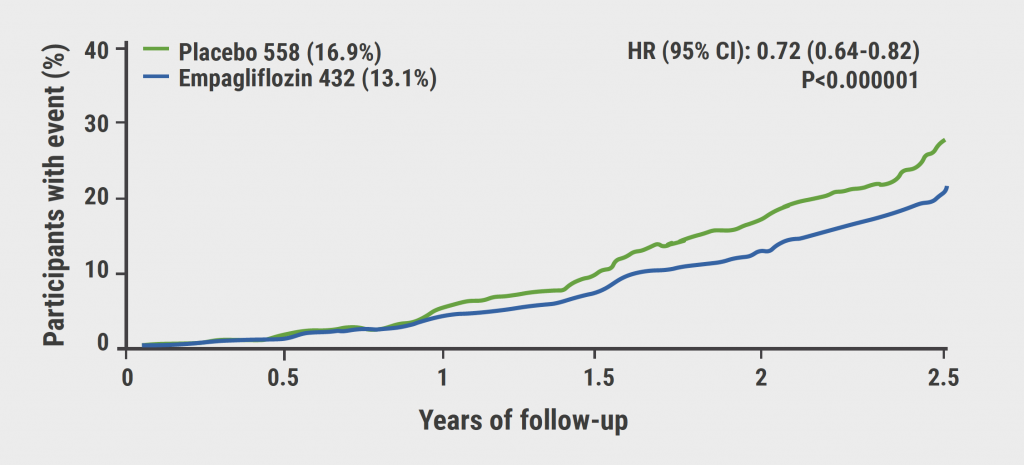
The ADU-CL-19 trial (NCT03945318) is an ongoing, phase 1/2 trial investigating zigakibart in participants with IgA nephropathy, a total protein excretion of ≥0.5 g/24h or 24-hour urine protein-creatinine ratio ≥0.5 g/g, and an eGFR ≥30 mL/min/1.73 m2. The trial enrolled two cohorts: Cohort 1 received intravenous zigakibart 450 mg every 2 weeks. After the new formulation became available (after 24 weeks, participants of this group switched to 600 mg zigakibart subcutaneously administered every 2 weeks. Cohort 2 received subcutaneously administered 600 mg zigakibart every 2 weeks. The primary endpoint was safety and tolerability, and 40 participants were included in the trial [1].
The 24-hour urine protein-creatinine ratio declined over the initial 52 weeks of therapy in both cohorts, reaching a mean -53.4% reduction. “For the treatment of IgA nephropathy, we aspire to maintain kidney function”, explained Prof. Jonathan Barrat (University of Leicester, United Kingdom). Indeed, zigakibart maintained eGFR levels over the first year of treatment in both the overall population of the trial and the individual cohorts. Zigakibart was overall well-tolerated. While adverse events (AEs) were observed, none led to treatment discontinuation or deaths. Most AEs were infectious events (as expected, as the trial was run during the COVID-19 pandemic). However, 7 participants also developed injection site reactions which were considered treatment-related. Only one serious AE was reported (amnesia) which was not considered to be treatment-related and did not lead to study drug discontinuation.
“In this trial, zigakibart reduced proteinuria, preserved kidney function in terms of a stable eGFR over the 52-week study period, and presented with an acceptable safety profile”, said Prof. Barrat. "We are currently evaluating zigakibart in the BEYOND phase 3 study (NCT05852938) which is open and recruiting”.
- Barrat J, et al. One year of zigakibart treatment shows clinically meaningful proteinuria reductioon and good tolerability in a Phase 1/2 study of IgA nephropathy. Abstract #55, ERA 2024, 23–26 May, Stockholm, Sweden.
Medical writing support was provided by Mihai Surducan, PhD.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Long-term atacicept shows continued benefit in IgA nephropathy Next Article
Atrasentan shows positive interim results in IgA nephropathy: ALIGN phase 3 trial »
« Long-term atacicept shows continued benefit in IgA nephropathy Next Article
Atrasentan shows positive interim results in IgA nephropathy: ALIGN phase 3 trial »
Table of Contents: ERA 2024
Featured articles
Meet the Experts: Navigating Kidneys and Genes
Chronic Kidney Disease
FLOW-trial: Semaglutide improves kidney and cardiovascular outcomes in type 2 diabetes and chronic kidney disease
Early phase data show albuminuria improvement with avenciguat
Rilparencel leads to kidney function stabilisation in chronic kidney disease and type 2 diabetes
The majority of real-world patients with CKD are not eligible for SGLT2 inhibitor trials
Kidney Transplantation and Dialysis
CD38 inhibition by felzartamab promising for resolution of antibody-mediated rejection following kidney allografts
TATH trial: Twice-weekly haemodialysis can be an alternative to thrice weekly regimen
KIR-HLA class I mismatch could be involved in antibody-mediated rejection of transplanted kidneys
IgA Nephropathy
Atrasentan shows positive interim results in IgA nephropathy: ALIGN phase 3 trial
Zigakibart slows down eGFR decline in IgA nephropathy
Long-term atacicept shows continued benefit in IgA nephropathy
APPLAUSE-IgAN: Iptacopan improves proteinuria in IgA nephropathy
Cardio-Renal Interplay
Semaglutide improves renal outcomes in overweight/obese participants with cardiovascular disease and no diabetes
Discrepancy between cardiovascular RCT participants and real-life CKD patients could limit generalisability of RCT results
MERCURI-1: Perioperative empagliflozin shows renal protection following cardiac surgery
Simulated head-to-head comparison of SGLT-2 inhibitors and GLP-1R agonists in type 2 diabetes
Other Nephrology
Preview of the new KDIGO Guidelines for ADPKD, available later in 2024
APPEAR-C3G: Iptacopan shows promise for complement 3 glomerulopathy
Anti-nephrin autoantibody positivity describes a unique subclass of podocytopathies
Active vitamin D plus low-dose prednisolone is an alternative to high-dose prednisolone in minimal change disease
Claudin-1 is a potential antibody target for crescent glomerulonephritis
Rituximab protocol based on PLA2R1 epitope spreading outperforms the standard GEMRITUX protocol in membranous nephropathy
The REACT score predicts relapse in ANCA-associated vasculitis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com