https://doi.org/10.55788/7dca8421
Golcadomide is an orally administered cereblon E3 ligase modulator, that degrades target proteins to produce dual immunomodulatory and direct cell-destructing anti-tumour activity [1]. The phase 1b CC-220-DLBCL-001 trial (NCT04884035) investigated the safety and efficacy of golcadomide plus R-CHOP in participants with untreated aggressive BCL. Safety was the primary endpoint of this study. Dr Marc Hoffman (The University of Kansas Cancer Center, KS, USA) presented the 12-month follow-up results [2].
Participants received ≤ 6 x 21-day cycles of golcadomide plus R-CHOP therapy and were randomised to 0.2 mg or 0.4 mg golcadomide at days 1 to 7 of each treatment cycle during the dose expansion phase. Of the 78 included participants, most had Ann Arbor stage III–IV (83.3%), 82% had high-risk disease, and 82% had diffuse large BCL with a histological diagnosis of “not otherwise specified”.
Grade 3 or 4 treatment-emergent adverse events (TEAEs) were seen in 91% of the participants, predominantly neutropenia (87%) and thrombocytopenia (42%). Serious TEAEs were reported in 46% of the participants, of which febrile neutropenia (19%) was the most common AE.
In the highest dose group (n=33), the CMR rate was 88% at the end of therapy and all participants were progression-free. This result was consistent across risk and cell of origin subgroups. At 12 months follow-up, the progression-free survival rate was 85% in the high-dose group and 75% in the low-dose group, with similar rates in biologically distinct molecular subtypes germinal centre B-cell (GCB) and activated B-cell (ABC; see Figure, diffuse large BCL only).
Figure: Treatment response and treatment follow-up per patient participant receiving the high golcadomide dose (0.4 mg) [2]
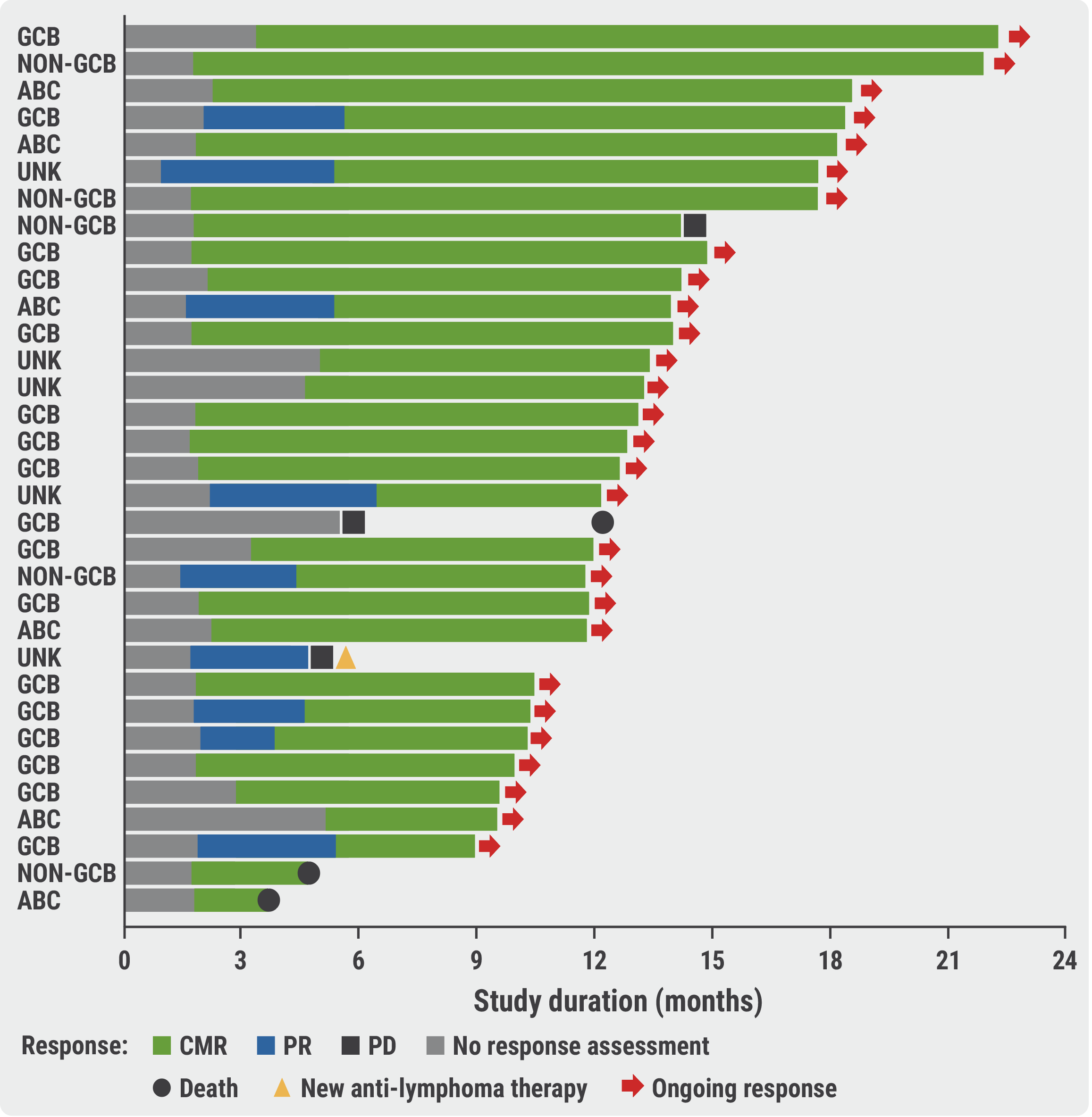
ABC, activated B-cell diffuse large BCL; CMR, complete metabolic response; (non-)GCB, (non-) germinal centre B-cell diffuse large BCL; PD, progressive disease; PR, partial response; UNK, unknown.
These findings support the currently recruiting GOLSEEK-1 trial (NCT06356129), an upstarting phase 3 study comparing golcadomide plus R-CHOP to R-CHOP alone as first-line treatment for participants with high-risk large BCL.
- Matyskiela ME, et al. J Med Chem. 2018;61(2):535–542.
- Hoffman M, et al. Golcadomide, a potential first-in-class oral CELMOD agent, plus R-chop in patients with untreated aggressive B-cell lymphoma: safety and 12-month efficacy results. S235, EHA congress 2024, 13–16 June, Madrid, Spain.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« High survival rates following atezolizumab consolidation in DLBCL Next Article
Is pelabresib plus ruxolitinib the paradigm-shifting combo therapy for myelofibrosis? »
« High survival rates following atezolizumab consolidation in DLBCL Next Article
Is pelabresib plus ruxolitinib the paradigm-shifting combo therapy for myelofibrosis? »
Table of Contents: EHA 2024
Featured articles
Meet the Expert: Prof. C. Ola Landgren discusses MRD as a key endpoint in haematological cancer trials
Multiple Myeloma
Isa-VRd proves its value in newly diagnosed MM in the IMROZ trial
PERSEUS: High MRD negativity rates with D-VRd and consolidation therapy and D-R maintenance in MM
Post-intensification data confirm superiority of quadruple therapy in MM
Promising phase 1 results for novel CAR T-cell therapy in MM
DREAMM 8: Belantamab mafodotin offers hope for patients with RRMM
Leukaemia
PhALLCON: Third-generation TKI superior to first-generation TKI in Ph+ ALL
APOLLO: ATRA plus ATO meets expectations in high-risk APL
Excellent phase 3 results for asciminib in chronic myeloid leukaemia
AUGMENT-101: Revumenib trial in KMT2Ar leukaemia stopped early for efficacy
FLAG-Ida plus venetoclax induces high MRD-negativity rates in AML
CD40/CD47 inhibitor shows promise in high-risk MDS and AML
ENHANCE: Magrolimab does not ameliorate health outcomes in high-risk MDS
Can MRD-guided azacitidine treatment improve outcomes in AML and MDS?
Can WGTS replace standard-of-care diagnostics in AML?
Non-malignant Haematology
ENERGIZE: Mitapivat meets primary efficacy endpoint in thalassaemia
Sovleplenib delivers durable responses and QoL improvements in primary ITP
Avatrombopag successful in children with chronic ITP
RUBY: Promising data for first AsCas12a gene-editing therapy in sickle cell disease
Encouraging data for ELA026 to treat secondary haemophagocytic lymphohistiocytosis
Myelofibrosis
Navitoclax plus ruxolitinib leads to spleen volume reductions in myelofibrosis
Is pelabresib plus ruxolitinib the paradigm-shifting combo therapy for myelofibrosis?
Lymphoma
The landscape of TP53 mutations and their prognostic impact in CLL
Can golcadomide plus R-CHOP become the first-line standard of care in high-risk BCL?
High survival rates following atezolizumab consolidation in DLBCL
First results for zanubrutinib plus venetoclax in del(17p)/TP53-mutated CLL/SLL
EPCORE CLL-1: Promising data for epcoritamab in high-risk Richter’s transformation
Updates from the EBMT Lymphoma Working Group: outcomes after allo- and auto-SCT for T-cell lymphoma subtypes
ECHO: Can we expect a novel standard of care in newly diagnosed MCL?
Clinically meaningful outcomes for mosunetuzumab across follicular lymphoma subgroups
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

