https://doi.org/10.55788/24af0499
“Novel therapies are needed in earlier lines of treatment for patients with MM,” stated Dr Paula Rodríguez-Otero (Clinica Universidad de Navarra, Spain) [1,2]. “The B-cell maturation antigen [BCMA]-directed CAR T-cell therapy ide-cel has displayed promising efficacy in a heavily pre-treated population of patients with RRMM, and we aimed to study this agent in earlier lines of therapy” [1,3]. The phase 3 KarMMa-3 trial (NCT03651128) compared ide-cel with standard treatment regimens in patients with triple-class exposed RRMM who had received 2 to 4 prior lines of therapy and were refractory to the last treatment regimen (n=386). The participants were randomised 2:1 to ide-cel or standard treatment. Progression-free survival (PFS) was the primary endpoint.
The median PFS was significantly longer in patients who received ide-cel compared with those who received a standard regimen (13.3 months vs 4.4 months; HR 0.49; 95% CI 0.38–0.65; P<0.0001; see Figure). “This result was consistent across subgroups, including older patients, those with a high tumour burden, and patients with high-risk cytogenetic abnormalities,” added Dr Rodríguez-Otero. Furthermore, the overall response rates (71% vs 42%; OR 3.47; P<0.0001) and the median duration of response (14.8 months vs 9.7 months) were higher in the ide-cel arm than in the control arm. Dr Rodriguez-Otero mentioned that the overall survival data were not yet mature at the time of the analysis. Finally, the safety data were consistent with the ide-cel toxicity profile reported in previous studies [1,4].
Figure: PFS in the intention-to-treat population of the KarMMa-3 study [1]
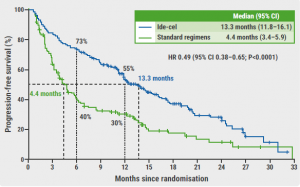
CI, confidence interval; HR, hazard ratio; ide-cel, idecabtagene vicleucel; PFS, progression-free survival.
In conclusion, the findings of the KarMMa-3 trial support the use of ide-cel in patients with early-line relapse and triple-class exposed RRMM, but OS data should be awaited.
- Rodriguez-Otero P, et al. Idecabtagene vicleucel versus standard regimens in patients with triple-class-exposed relapsed and refractory multiple myeloma: KarMMa-3, a phase 3 randomized controlled trial. GS02-10, European Society for Blood and Marrow Transplantation (EBMT) 49th Annual Meeting, 23–26 April 2023, Paris, France.
- Rodríguez-Otero P, et al. N Engl J Med 2023;388:1002–1014.
- Munshi NC, et al. N Engl J Med 2021;384:705–716.
- Raje N, et al. N Engl J Med 2019;380:1726–1737.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Letter from the Editor Next Article
New developments in steroid-refractory acute GvHD »
« Letter from the Editor Next Article
New developments in steroid-refractory acute GvHD »
Table of Contents: EBMT 2023
Featured articles
CAR T cells rise to the front in multiple myeloma
Acute Leukaemia
Quizartinib plus chemotherapy improves OS in patients with AML undergoing ASCT
Blinatumomab may improve outcomes in patients with B-cell ALL undergoing ASCT
Is ASCT a reasonable option in patients with invasive aspergillosis?
Tacrolimus versus cyclosporine A in AML
Promising novel target identified for AML
Multiple Myeloma
Ide-cel superior to standard therapies in triple-class exposed RRMM
ASCT or CAR T cell as first-line therapy for MM?
DETERMINATION: Does one size fit all in multiple myeloma?
Graft-Versus-Host Disease
New options to treat steroid-refractory chronic GvHD
New developments in steroid-refractory acute GvHD
Miscellaneous Topics
Long-term success for CD19 CAR T-cell therapy in CLL
Can molecular data improve prognostication in MDS patients undergoing HSCT?
Next-generation cell therapies for cancer: CAR-NK cells
Novel drugs and strategies around ASCT for Hodgkin lymphoma
Thalassaemia: Advances in conventional transplantation and gene therapy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy