https://doi.org/10.55788/107fc782
The results of the phase 3 BMT CTN 1703 trial (NCT03959241) were presented by Prof. Shernan Holtan (University of Minnesota, MN, USA) [1]. The study included adult patients receiving RIC ASCT (n=431), who were randomised 1:1 to post-transplant PTCy/Tac/MMF or Tac/MTX. The primary endpoint was the 1-year GVHD-free relapse-free survival (GRFS), a composite endpoint of grade III–IV acute GVHD, chronic GVHD requiring systemic immunosuppression, relapse/progression, or death.
The primary endpoint was met, with a 1-year GRFS of 52.7% in the PTCy/Tac/MMF arm and a 1-year GRFS of 34.9% in the Tac/MTX arm (HR 0.64; P<0.001). The effect was driven by a reduction in grade III–IV acute GVHD (6.3% vs 14.7%, respectively; P=0.001) and a decrease in chronic GVHD requiring systemic immunosuppression (12.5% vs 25%, respectively; P=0.001). Prof. Holtan added that relapse/progression rates were similar, with 20.8% in the PTCy/Tac/MMF arm and 20.2% in the Tac/MTX arm (P=0.906; see Figure).
Figure: Improved GVHD outcomes not at expense of relapse [1]
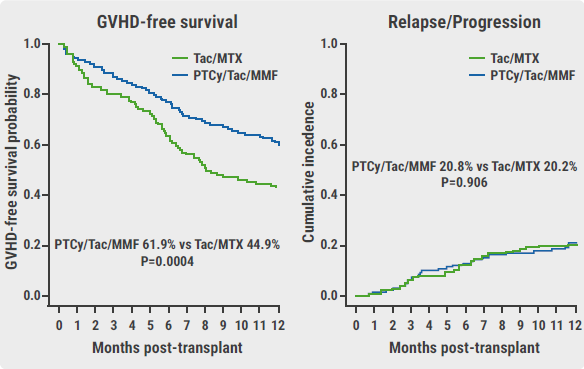
GVHD, graft-versus-host disease; PTCy/Tac/MMF, post-transplant cyclophosphamide/tacrolimus/mycophenolate mofetil; Tac/MTX, tacrolimus/methotrexate.
There were more grade 2–3 infections in the experimental arm (40.0% vs 30.4; P=0.018), mostly explained by an increased rate of grade 2 infections. Also, fewer patients in the experimental arm achieved an absolute lymphocyte count >1,000 (53.8% vs 63.2%; P<0.001). Finally, after 1 year of follow-up, it appeared that patients in the Tac/MTX arm were more likely to die from acute GVHD (14.3% vs 4.2%), whereas patients in the PTCy/Tac/MMF arm were more likely to die from organ failure (22.9% vs 10.7%).
The authors concluded that PTCy/Tac/MMF should become the standard-of-care GVHD prophylaxis in adults receiving RIC ASCT.
- Holtan S, et al. BMT CTN 1703: A randomized, Multicenter, Phase III Trial of Tacrolimus/Methotrexate versus Post-Transplant Cyclophosphamide/Tacrolimus/Mycophenolate Mofetil in Non-Myeloablative/Reduced Intensity Conditioning Allogeneic Peripheral Blood Stem Cell Transplantation. Late-Breaking Abstract 4, ASH 64th Annual Meeting, 10–13 December 2022, New Orleans, LA, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« LMWH does not result in higher live birth rates in women with inherited thrombophilia Next Article
Iptacopan offers solution for patients with PNH and residual anaemia after standard-of-care »
« LMWH does not result in higher live birth rates in women with inherited thrombophilia Next Article
Iptacopan offers solution for patients with PNH and residual anaemia after standard-of-care »
Table of Contents: ASH 2022
Featured articles
Acute Lymphoblastic Leukaemia
Blinatumomab candidate for standard-of-care in newly diagnosed B-ALL
High-dose methotrexate or standard interim maintenance in young patients with ALL?
Acute Myeloid Leukaemia
Excellent results for triplet regimen in FLT3-mutated AML
MRD by qPCR prognostic of outcomes in venetoclax-treated NPM1-mutated AML
Promising results for triplet therapy with magrolimab in AML
Should we use intensive chemotherapy prior to allo-HCT in relapsed/refractory AML?
Chronic Leukaemia
Zanubrutinib wins battle of BTK inhibitors in relapsed or refractory CLL/SLL
Ibrutinib plus venetoclax displays long-term benefits in CLL
Multiple Myeloma
Talquetamab further investigated in heavily pre-treated MM after promising phase 2 data
Promising results of elranatamab for MM in phase 2 MagnetisMM-3 trial
Deep and durable responses for quadruple therapy in smouldering MM
Ultra-sensitive MRD assessment in MM with BloodFlow
CAR-Hematotox score proves useful in relapsed/refractory MM
Head-to-head: VMP versus Rd in transplant-ineligible MM
Lymphoma
Ibrutinib added to ASCT improves clinical outcomes in mantle cell lymphoma
High-dose chemotherapy plus ASCT superior to standard immuno-chemotherapy in primary CNS lymphoma
Odronextamab has considerable anti-tumour effects in relapsed/refractory diffuse large B-cell lymphoma and follicular lymphoma
Excellent results for AFM13-complexed NK cells in CD30-positive lymphoma
CAR-Hematotox score predicts toxicity, infections, and clinical outcomes in MCL
Myeloproliferative Neoplasms
Efgartigimod successful in immune thrombocytopenia
INCA033989: novel investigational agent for CALR-mutated MPN
Ruxolitinib mediates clonal evolution of RAS pathway mutations in MPN
Immune Thrombocytopenia
Long-term risk for haematologic disease in persistent, isolated mild thrombocytopenia
Various Topics
C1 inhibitor deficiency linked to thrombosis
Durable responses to gene therapy in haemophilia A
Long-term benefits from beti-cel in transfusion-dependent β-thalassaemia
Neutrodiet: non-restricted diet is the preferred option after SCT
Iptacopan offers solution for patients with PNH and residual anaemia after standard-of-care
Novel therapy may replace standard-of-care prophylaxis for GVHD
LMWH does not result in higher live birth rates in women with inherited thrombophilia
Related Articles
February 20, 2023
Long-term benefits from beti-cel in transfusion-dependent β-thalassaemia
February 20, 2023
C1 inhibitor deficiency linked to thrombosis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

