The VEDOKIDS study (NCT02862132), a prospective cohort study, included 142 children (0–18 years) with Crohn’s disease (CD; n=65) or ulcerative colitis (UC; n=77), of whom approximately 68% had failed on previous anti-TNF treatment [1]. The participants were exposed to intravenous vedolizumab 177 mg/m2 Body Surface Area (BSA) up to a maximum of 300 mg at weeks 0, 2, 6 and then every 8 weeks. Clinical remission, defined as steroid-free and exclusive enteral nutrition (EEN)-free remission, was the main clinical outcome of the study. The results at week 14 were presented by Dr Dan Turner (Juliet Keidan Institute of Paediatric Gastroenterology and Nutrition, Israel).
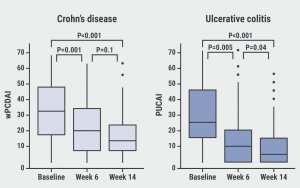
At week 14, 40% of the participants with CD achieved clinical remission and 21% reached steroid-free remission with normal erythrocyte sedimentation rate (ESR)/C-reactive protein (CRP). For participants with UC, the corresponding figures were 51% and 36%. Moreover, significant treatment effects were already observed at week 6 (see Figure). Clinical remission proportions in participants receiving vedolizumab as first-line or second-line therapy were not significantly different, suggesting a comparable efficacy of vedolizumab in biologic-naïve participants and participants who failed on a biologic therapy. Notably, disease activity at baseline, measured by the Pediatric Ulcerative Colitis Activity Index (PUCAI), was predictive of clinical remission at week 14 (AUC 0.66; 95% CI 0.54–0.79). Moreover, Mucosal Inflammation Non-invasive (MINI) index (AUC 0.79; 95% CI 0.64–0.94) and weighted Pediatric Crohn's Disease Activity Index (wPCDAI) scores (AUC 0.70; 95% CI 0.57–0.84) were predictive of clinical remission. Dr Turner added that children >30 kg could be dosed as adults (300 mg), whereas children <30 kg may be dosed with 200 mg or 10 mg/kg.
Figure: Rapid improvement in clinical activity [1]
In total, 114 adverse events were reported. Of these adverse events, 32 were possibly related to vedolizumab therapy. Fortunately, all of those events were mild or moderate. Only 2 adverse event-related treatment discontinuations were reported: 1 case of leukocytoclastic vasculitis and 1 case of dyspnoea.
- Shavit Z, et al. Outcome of induction therapy with vedolizumab in children: Results from the prospective, multi-centre VEDOKIDS study. OP05, ECCO 2022, 16–19 February.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Primary endpoint of 5-hydroxytryptophan for fatigue in IBD not met Next Article
Vedolizumab first approved therapy for chronic pouchitis »
« Primary endpoint of 5-hydroxytryptophan for fatigue in IBD not met Next Article
Vedolizumab first approved therapy for chronic pouchitis »
Table of Contents: ECCO 2022
Featured articles
Upadacitinib maintenance therapy delivers sustained improvements in active ulcerative colitis
Novel Treatment Modalities
Guselkumab shows encouraging safety and efficacy in ulcerative colitis
Guselkumab maintenance therapy achieved high efficacy rates in Crohn’s disease
Mirikizumab efficacious for active ulcerative colitis
Risankizumab more efficacious in colonic than in ileal Crohn’s disease
Guselkumab plus golimumab promising combination for ulcerative colitis
Combined endpoint may support personalised medicine in ulcerative colitis
Filgotinib seems promising for perianal fistulising Crohn’s disease
Upadacitinib maintenance therapy delivers sustained improvements in active ulcerative colitis
Upadacitinib counters extraintestinal manifestations in ulcerative colitis
Deucravacitinib does not meet primary endpoint for ulcerative colitis
Head-to-Head Comparisons
Anti-TNFs versus vedolizumab and ustekinumab in Crohn’s disease
Upadacitinib appears to be an efficacious therapy for moderately-to-severely ulcerative colitis
Subcutaneous infliximab versus subcutaneous vedolizumab in IBD
Vedolizumab outperforms anti-TNF in biologic-naïve ulcerative colitis
Short-Term and Long-Term Treatment Results
Ozanimod treatment shows maintained response in ulcerative colitis
Stopping infliximab but not antimetabolites leads to more relapses in Crohn’s disease
Vedolizumab first approved therapy for chronic pouchitis
VEDOKIDS: Vedolizumab seems effective in paediatric IBD
Primary endpoint of 5-hydroxytryptophan for fatigue in IBD not met
Specific Therapeutic Strategies
Positive outcomes with therapeutic drug monitoring during infliximab maintenance therapy
Segmental colectomy beneficial over total colectomy in Chrohn’s disease
Modified 2-stage ileal pouch-anal anastomosis versus 3-stage alternative
Similar results for different corticosteroid tapering protocols in UC
Miscellaneous Topics
Lessons from the COVID-19 pandemic for IBD management
AI model distinguishes between histologic activity and remission in ulcerative colitis
Multi-Omic and dietary analysis of Crohn’s disease identifies pathogenetic factors
Novel classification system for perianal fistulising Crohn’s disease
Vaccination tool associated with improved vaccination coverage in IBD
Comparable safety profiles of biological therapies in elderly patients with IBD
Early biologic therapy induces larger effect than delayed treatment in Crohn’s disease
RESTORE-UC: No better outcomes with FMT superdonors than with autologous stools
Related Articles
May 9, 2019
Pan-Janus kinase inhibitor TD-1473
October 27, 2021
Upadacitinib efficacious and safe as maintenance therapy for UC
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

