In this episode (17:15 min), Medicom’s correspondent covers 6 presentations from the 17th Congress of the European Crohn’s and Colitis Organisation (ECCO’22), which was held as a virtual event from 16-19 February 2022.
- Mirikizumab safe and efficacious for active UC
Mirikizumab was more efficacious than placebo as induction therapy for participants with active ulcerative colitis (UC). In addition, the safety profile of mirikizumab was favourable. These results from the phase 3 LUCENT-1 trial support the applicability of mirikizumab in patients with UC. - Upadacitinib maintenance therapy delivers sustained improvements in active UC
Upadacitinib maintenance therapy was associated with sustained improvements in abdominal pain, bowel urgency, and fatigue in patients with moderately to severely active ulcerative colitis (UC) who responded to upadacitinib induction therapy. Numerical benefits were observed for the high-dose maintenance group over the low-dose maintenance group. These are the main results of a study investigating secondary endpoints of the U-ACHIEVE maintenance trial. - Vedolizumab first approved therapy for chronic pouchitis
Vedolizumab showed clinical, endoscopic, and histologic benefits over placebo in patients with chronic pouchitis after ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC). The safety profile of vedolizumab was consistent with previous data published on this agent. The phase 4 EARNEST trial is the first and largest randomised controlled trial to demonstrate significant benefits of a biologic therapy in patients with chronic pouchitis. - Benefits of segmental colectomy over total colectomy
Segmental colectomy (SC) was not associated with an increased risk of surgical recurrence compared with total colectomy (TC) in participants with Crohn’s disease (CD). In addition, SC did reduce the risk of a temporary or permanent stoma. The large-scale international, multicentre SCOTCH study adds high-quality data to the understanding of colectomy in CD. - Stopping infliximab, but not anti-metabolites, leads to more relapses in CD
In patients with Crohn’s disease (CD) who achieved sustained remission on infliximab plus anti-metabolite therapy, infliximab discontinuation was associated with an increased risk of relapse. In contrast, anti-metabolite discontinuation did not lead to significantly higher relapse rates compared to continuation of the combination treatment. Since physicians often contemplate de-escalation of infliximab plus anti-metabolite therapy, these results may add to the decision-making process of treatment de-escalation in patients with CD. - RESTORE-UC: No better outcomes with FMT superdonors than with autologous stools
The use of superdonor stools for faecal microbiota transplantation (FMT) did not outperform autologous stools in initiating remission in patients with active ulcerative colitis (UC). Furthermore, the current RESTORE-UC trial presented an FMT anaerobic preparation and administration protocol to improve the international standardisation of this therapy.
Enjoy listening!
Posted on
Previous Article
« IV gentamicin shows promise for genetic blistering skin disorder Next Article
COVID-19 can cause brain shrinkage, memory loss – study »
« IV gentamicin shows promise for genetic blistering skin disorder Next Article
COVID-19 can cause brain shrinkage, memory loss – study »
Table of Contents: ECCO 2022
Featured articles
Upadacitinib maintenance therapy delivers sustained improvements in active ulcerative colitis
Novel Treatment Modalities
Guselkumab shows encouraging safety and efficacy in ulcerative colitis
Guselkumab maintenance therapy achieved high efficacy rates in Crohn’s disease
Mirikizumab efficacious for active ulcerative colitis
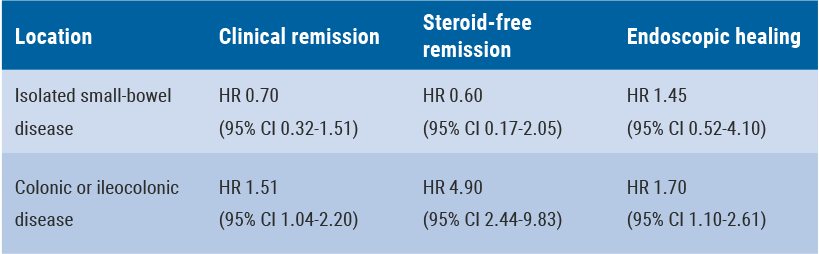
Risankizumab more efficacious in colonic than in ileal Crohn’s disease
Guselkumab plus golimumab promising combination for ulcerative colitis
Combined endpoint may support personalised medicine in ulcerative colitis
Filgotinib seems promising for perianal fistulising Crohn’s disease
Upadacitinib maintenance therapy delivers sustained improvements in active ulcerative colitis
Upadacitinib counters extraintestinal manifestations in ulcerative colitis
Deucravacitinib does not meet primary endpoint for ulcerative colitis
Head-to-Head Comparisons
Anti-TNFs versus vedolizumab and ustekinumab in Crohn’s disease
Upadacitinib appears to be an efficacious therapy for moderately-to-severely ulcerative colitis
Subcutaneous infliximab versus subcutaneous vedolizumab in IBD
Vedolizumab outperforms anti-TNF in biologic-naïve ulcerative colitis
Short-Term and Long-Term Treatment Results
Ozanimod treatment shows maintained response in ulcerative colitis
Stopping infliximab but not antimetabolites leads to more relapses in Crohn’s disease
Vedolizumab first approved therapy for chronic pouchitis
VEDOKIDS: Vedolizumab seems effective in paediatric IBD
Primary endpoint of 5-hydroxytryptophan for fatigue in IBD not met
Specific Therapeutic Strategies
Positive outcomes with therapeutic drug monitoring during infliximab maintenance therapy
Segmental colectomy beneficial over total colectomy in Chrohn’s disease
Modified 2-stage ileal pouch-anal anastomosis versus 3-stage alternative
Similar results for different corticosteroid tapering protocols in UC
Miscellaneous Topics
Lessons from the COVID-19 pandemic for IBD management
AI model distinguishes between histologic activity and remission in ulcerative colitis
Multi-Omic and dietary analysis of Crohn’s disease identifies pathogenetic factors
Novel classification system for perianal fistulising Crohn’s disease
Vaccination tool associated with improved vaccination coverage in IBD
Comparable safety profiles of biological therapies in elderly patients with IBD
Early biologic therapy induces larger effect than delayed treatment in Crohn’s disease
RESTORE-UC: No better outcomes with FMT superdonors than with autologous stools
Related Articles
April 12, 2022
Vedolizumab first approved therapy for chronic pouchitis
March 12, 2021
Olamkicept may induce response in IBD patients
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

