https://doi.org/10.55788/99fdcc84
Prof. Simon Travis (University of Oxford, UK) shared patient-reported outcomes of patients with CD who received mirikizumab (n=579) or a placebo (n=199) in the phase 3 VIVID-1 study (NCT03926130) [1]. Fatigue was measured with the FACIT-fatigue instrument, bowel urgency was assessed with a numeric rating scale, and the Inflammatory Bowel Disease Questionnaire (IBDQ) was used to evaluate health-related QoL.
After 12 weeks of therapy, participants in the mirikizumab arm had an average of 5.86 points improvement in fatigue compared with an average of 2.64 points in participants on placebo (P<0.0001). At week 52, the corresponding figures were 7.47 and 3.08 (P<0.0001). “Already after 12 weeks, patients in the mirikizumab arm achieved the 5-point improvement that is considered a clinically meaningful difference,” emphasised Prof. Travis.
Furthermore, bowel urgency was significantly improved among participants receiving mirikizumab compared with those receiving a placebo at week 12 (-2.44 vs -1.58; P<0.0001) and week 52 (-3.24 vs -1.23; P<0.0001). Finally, the IBDQ scores showed that health-related QoL had also improved in the experimental arm compared with the placebo arm at week 12 (+36.9 vs + 17.4; P<0.000001) and week 52 (+43.8 vs +15.9; P<0.000001). “These improvements were seen across domain scores, such as bowel symptoms, systemic symptoms, emotional function, and social function,” added Prof. Travis (see Figure).
Figure: IBDQ total and domain scores of mirikizumab versus placebo at week 12 and week 52 [1]
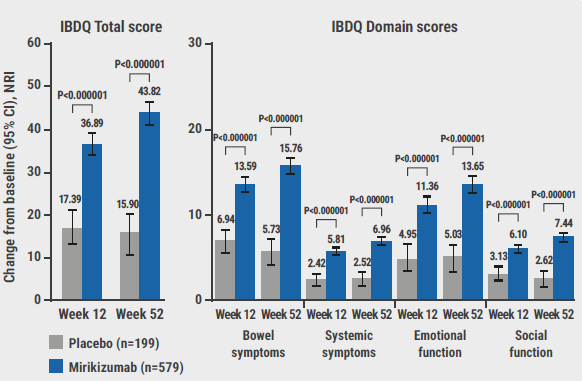
CI, confidence interval; IBDQ, Inflammatory Bowel Disease Questionnaire; NRI, non-responder imputation.
In conclusion, in the VIVID-1 trial, mirikizumab was superior to placebo in terms of improving fatigue, bowel urgency, and health-related QoL in patients with CD.
- Travis S, et al. Mirikizumab improves fatigue bowel urgency, and quality of life in patients with moderately to severely active Crohn’s disease: results from a phase 3 clinical trial. OP12, 19th Congress of ECCO, 21–24 February 2024, Stockholm, Sweden.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Novel agent VTX002 holds promise in ulcerative colitis Next Article
QUASAR: Guselkumab improves QoL for patients with ulcerative colitis »
« Novel agent VTX002 holds promise in ulcerative colitis Next Article
QUASAR: Guselkumab improves QoL for patients with ulcerative colitis »
Table of Contents: ECCO 2024
Featured articles
Meet the Trialist: Dr Yasuharu Maeda on AI-assisted endoscopy
IL-23 Inhibitors on the Rise
VIVID-1: Mirikizumab meets expectations in Crohn’s disease
COMMAND: Long-term efficacy benefits of risankizumab in ulcerative colitis
SEQUENCE: Risankizumab versus ustekinumab across endpoints
QUASAR: Guselkumab improves QoL for patients with ulcerative colitis
Fatigue, urgency, and QoL improvements on mirikizumab in Crohn’s disease
Inspiring Drug Trials and Treatment Strategies
Novel agent VTX002 holds promise in ulcerative colitis
PROFILE: Top-down treatment strategy benefits patients with early Crohn’s disease
Biologicals and JAK inhibitors hold promise in microscopic colitis
Ustekinumab as alternative for anti-TNFs in HLA-DQA1*05-positive Crohn’s disease
How effective is dose escalation of biologicals in IBD?
Make Way for JAK Inhibitors
Promising data for JAK inhibitors in Crohn’s disease from phase 2 trial
U-ENDURE long-term extension: sustained efficacy of upadacitinib in Crohn’s disease
TRIUMPH: Tofacitinib as rescue option for acute severe ulcerative colitis
Focus on Endoscopy, Screening, and Risk Factors
Should we screen for metabolic bone disease at IBD diagnosis?
Predicting relapse in ulcerative colitis with AI-assisted endoscopy
Clear case for NUDT15 genetic testing in Asian patients with IBD
HELIOS: HD-WLE can yield similar neoplasia detection rates as HD-CE
CURE-CD: Capsule endoscopy-guided proactive treatment leads to fewer relapses in Crohn’s disease
Sharp Surgical Solutions
Extended mesenterectomy or mesenteric-sparing surgery in Crohn’s disease?
Similar outcomes for Kono-S and side-to-side anastomosis in Crohn’s terminal ileitis
Risk factors for re-resection in Crohn’s disease revealed
ADMIRE-CD-II: Darvadstrocel does not meet primary endpoint in complex peri-anal fistula
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com