https://doi.org/10.55788/c744371e
INSPIRE (NCT03398148), a previous phase 3 induction trial, demonstrated the superiority of the IL-23 inhibitor risankizumab (1,200 mg i.v.) over placebo in terms of clinical and endoscopic endpoints in patients with UC [1]. The current phase 3 COMMAND trial (NCT03398135) enrolled 588 patients with UC who responded to the 12-week induction regimen of INSPIRE [2]. These participants were randomised 1:1:1 to risankizumab, either 180 mg or 360 mg s.c., every 8 weeks, or a placebo. Clinical remission (defined as modified Mayo score [MMS] stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore =0, and endoscopic subscore ≤1) at week 52 was the primary endpoint, and Prof. Stefan Schreiber (University Hospital Schleswig-Holstein, Germany) presented the findings.
At week 52, 40.2% and 37.6% of the participants in the 180 mg and 360 mg groups achieved clinical remission, compared with 25.1% of the participants on placebo, significantly favouring the experimental arms over the control arm. This difference appeared to be more pronounced among adequate responders to prior advanced therapy (50.9% in the 180 mg group, 61.7% in the 360 mg group, vs 31.1% on placebo) but was still present among participants who did show an inadequate response to a previous advanced therapy (36.6% in the 180 mg group, 29.5% in the 360 mg group, vs 23.2% on placebo). Endoscopic, histologic, patient-reported, and more stringent clinical endpoints all favoured risankizumab over placebo in this population (see Figure). Lastly, the drug was well tolerated and the safety profile was consistent with previously published data.
Figure: Histologic and endoscopic endpoints at week 52 in the COMMAND trial [2]
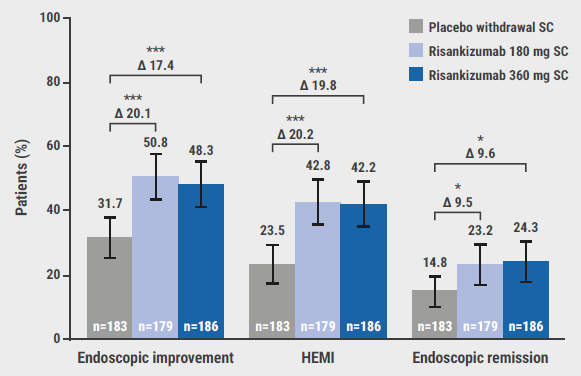
HEMI, Histological-endoscopic mucosal improvement; SC, subcutaneous.
“Risankizumab maintenance therapy was superior to placebo withdrawal treatment in patients with UC who responded to risankizumab induction therapy, without substantial toxicity,” concluded Prof. Schreiber.
- Louis et al. Abstract OP021, UEG Journal. 2023;11(S8).
- Schreiber S, et al. Risankizumab maintenance therapy in patients with moderately to severely active ulcerative colitis: efficacy and safety in the randomized phase 3 COMMAND study. OP06, 19th Congress of ECCO, 21–24 February 2024, Stockholm, Sweden.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« SEQUENCE: Risankizumab versus ustekinumab across endpoints Next Article
VIVID-1: Mirikizumab meets expectations in Crohn’s disease »
« SEQUENCE: Risankizumab versus ustekinumab across endpoints Next Article
VIVID-1: Mirikizumab meets expectations in Crohn’s disease »
Table of Contents: ECCO 2024
Featured articles
Meet the Trialist: Dr Yasuharu Maeda on AI-assisted endoscopy
IL-23 Inhibitors on the Rise
VIVID-1: Mirikizumab meets expectations in Crohn’s disease
COMMAND: Long-term efficacy benefits of risankizumab in ulcerative colitis
SEQUENCE: Risankizumab versus ustekinumab across endpoints
QUASAR: Guselkumab improves QoL for patients with ulcerative colitis
Fatigue, urgency, and QoL improvements on mirikizumab in Crohn’s disease
Inspiring Drug Trials and Treatment Strategies
Novel agent VTX002 holds promise in ulcerative colitis
PROFILE: Top-down treatment strategy benefits patients with early Crohn’s disease
Biologicals and JAK inhibitors hold promise in microscopic colitis
Ustekinumab as alternative for anti-TNFs in HLA-DQA1*05-positive Crohn’s disease
How effective is dose escalation of biologicals in IBD?
Make Way for JAK Inhibitors
Promising data for JAK inhibitors in Crohn’s disease from phase 2 trial
U-ENDURE long-term extension: sustained efficacy of upadacitinib in Crohn’s disease
TRIUMPH: Tofacitinib as rescue option for acute severe ulcerative colitis
Focus on Endoscopy, Screening, and Risk Factors
Should we screen for metabolic bone disease at IBD diagnosis?
Predicting relapse in ulcerative colitis with AI-assisted endoscopy
Clear case for NUDT15 genetic testing in Asian patients with IBD
HELIOS: HD-WLE can yield similar neoplasia detection rates as HD-CE
CURE-CD: Capsule endoscopy-guided proactive treatment leads to fewer relapses in Crohn’s disease
Sharp Surgical Solutions
Extended mesenterectomy or mesenteric-sparing surgery in Crohn’s disease?
Similar outcomes for Kono-S and side-to-side anastomosis in Crohn’s terminal ileitis
Risk factors for re-resection in Crohn’s disease revealed
ADMIRE-CD-II: Darvadstrocel does not meet primary endpoint in complex peri-anal fistula
Related Articles
August 18, 2021
Certain antibiotics may drive anti-TNF immunogenicity in IBD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

