Researchers examined data from 52 randomized controlled trials of oral and biologic medicines for moderate to severe plaque psoriasis. The analysis included 52 trials that looked at short-term safety outcomes (12 to 16 weeks from baseline) for 16 treatments, and it also included seven trials with long-term safety outcomes (48 to 56 weeks from baseline) for 7 treatments.
With short-term usage, the treatments associated with the lowest rates of any adverse events were tildrakizumab (46% for 200mg Q12W), certolizumab (46.2% for 200 mg Q2W), entanercept (49.1%), risankizumab (52.4%), and guselkumab (55.8%). When researchers looked just at serious adverse events, the treatments with the lowest rates for short-term use were certolizumab (0.8% for 200 mg Q2W), risankizumab (1.2%), entanercept (1.6%), and dimethyl fumarate (1.8%).
Risankizumab had the lowest odds of adverse events leading to treatment discontinuation after short-term use (0.5%).
With long-term usage, risankizumab had the lowest rates of any adverse events (67.5%), serious adverse events (4.4%), and treatment discontinuation due to adverse events (1.0%).
"Based on the clinical trial results, our relatively newer class of biologics IL-23 have a favorable safety profile, and this is likely due to the precision by which we dampen the part of the inflammation system responsible for psoriasis," said senior study author Dr. April Armstrong, a professor of dermatology and associate dean of clinical research at the Keck School of Medicine at the University of Southern California, in Los Angeles.
"In addition, the dosing of the biologics for psoriasis are carefully calculated such that, in general, in most patients, these medications help to reduce the excess cytokines that are elevated in psoriasis skin, but they also don't tend to over-correct that," Dr. Armstrong said by email.
With long-term use, the study found the second-lowest rate of any adverse events with guselkumab (72.2%), which also had the third-lowest rate of treatment discontinuation due to adverse events (2.5%).
One limitation of the study is that results from the clinical trials used in the analysis might not be representative of outcomes in real-world use, the study team notes in the Journal of the American Academy of Dermatology. It also wasn't possible with the available data to examine rates of specific adverse events such as infections.
Trial data is not fully reflective of the clinic, and the potential safety benefits seen in the current analysis should be confirmed in safety registries, said Dr. Zenas Yiu, a clinical lecturer in dermatology at the University of Manchester, in the UK, who wasn't involved in the study.
"It is also unclear why risankizumab has the most favorable benefit-risk profile from trial data when compared to other IL-23 inhibitors, and it is likely down to subtle differences in binding affinity and antibody structure that differentiate these therapies," Dr. Yiu said by email.
Clinicians, however, should be reassured that IL-23 inhibitors don't appear worse and may potentially be better than other options, Dr. Yiu said.
"I think clinicians should continue to use decision aids that take into account safety and benefit of therapies as well as patient values, frequency of treatment and presence of long-term safety data prior to prescribing a certain therapy," Dr. Yiu added.
SOURCE: https://bit.ly/3coI8Kh Journal of the American Academy of Dermatology, online February 22, 2021.
By Lisa Rapaport
Posted on
Previous Article
« Outcomes with COVID-19 acute kidney injury worse than for other AKI patients Next Article
IBD associated with high-grade cervical neoplasia in women »
« Outcomes with COVID-19 acute kidney injury worse than for other AKI patients Next Article
IBD associated with high-grade cervical neoplasia in women »
Related Articles
May 9, 2019
IBD risk of treatment with IL-17 antagonists
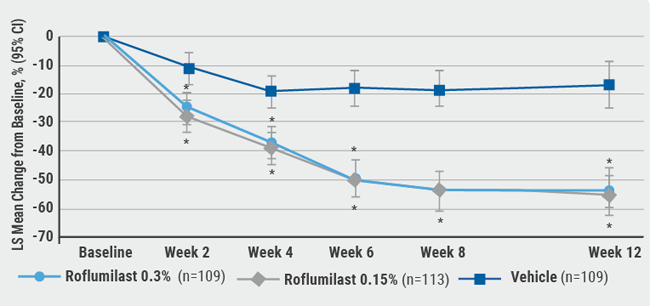
August 6, 2020
A new topical PDE-4 inhibitor effective in psoriasis
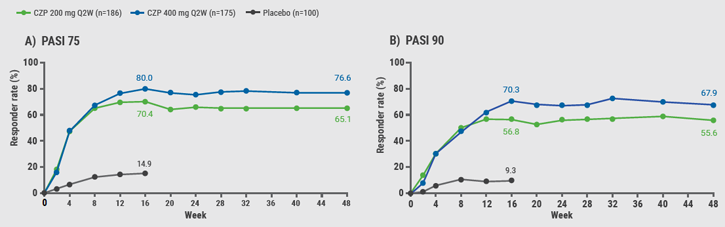
October 9, 2019
Certolizumab pegol efficacious for head and neck psoriasis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

