Alopecia areata (AA) frequently causes significant emotional and psychosocial distress. At present, there is no FDA-approved treatment for AA. In AA, loss of hair follicle immune privilege leads to infiltration by CD8-positive natural killer cells that secrete interferon-γ, which signals through JAK1/2 in the hair follicle epithelial cells [1]. This process is followed by hair follicle damage and hair loss.
Baricitinib is an oral, selective JAK1/2 inhibitor that has demonstrated superiority over placebo in hair regrowth in the phase 2 portion of the BRAVE-AA1 study (NCT03570749) [2]. Prof. Brett King (Yale University School of Medicine, CT, USA) presented the 36-week results of the phase 3 portion of BRAVE-AA1 and the phase 3 trial BRAVE-AA2 (NCT03899259), which were simultaneously published [3].
The participants (BRAVE-AA1, n=654 and BRAVE-AA2, n=546) were adults with severe or very severe AA (defined by Severity of Alopecia Tool [SALT] score 50–94 or 95–100, respectively). The SALT score is a weighted sum of the percentage of hair loss in the 4 quadrants of the scalp, ranging from 0 (i.e. no hair loss) to 100 (i.e. complete hair loss). “We chose an upper age of 60 in men to minimise effects of androgenetic alopecia,” Prof. King explained. The duration of AA was similar between treatment groups. The mean SALT score was 85 across the treatment groups, and 40% to 47% of the participants had alopecia universalis. In both trials, patients with AA were randomised to placebo or baricitinib 2 mg or 4 mg once daily. The primary endpoint was the proportion of patients that achieved a SALT score ≤20 at week 36.
In BRAVE-AA1, the primary endpoint was achieved by 35.2% of participants on 4 mg baricitinib and 21.7% on 2 mg baricitinib. The corresponding numbers in the BRAVE-AA2 trial were 32.5% and 17.3%, respectively (see Figure). Likewise, significant regrowth of brows and lashes were seen in the groups treated with low-dose baricitinib.
Figure: Proportion of patients achieving SALT score ≤20 through week 36 (primary study endpoint) [3]
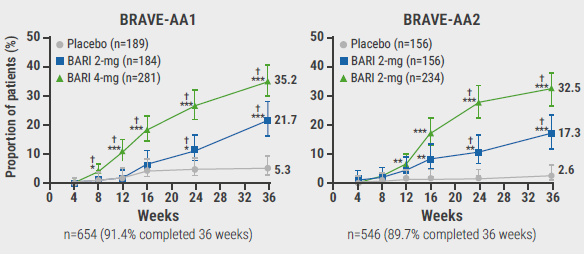
†Statistically significant compared with placebo after multiplicity adjustment in graphical testing schemes;*P<0.05, **P<0.01, ***P<0.001 versus placebo without adjustment for multiple comparisons.
Baricitinib was well tolerated. No new safety findings were identified compared with the known safety profile. The number of treatment-emergent adverse events were mild to moderate in most cases and there was no death. Common adverse events in both trials included upper respiratory tract infections, nasopharyngitis, headaches, urinary tract infections, and elevated creatine phosphokinase. There was one major adverse cardiovascular event in the baricitinib 2 mg group in a patient with multiple cardiovascular risk factors. Of note, no venous thromboembolic events were reported in either study.
Prof. King concluded that longer periods of observation are required to evaluate the long-term clinical response and safety.
- Islam N, et al. Autoimmun Rev. 2015;14:81–89.
- King B, et al. J Am Acad Dermatol Venereol. 2021;85:847–53.
- King B. Efficacy and safety of baricitinib in adults with alopecia areata: Phase 3 results from two randomized controlled trials (BRAVE-AA1 and BRAVE-AA2). FC02.05, EADV Congress 2021, 29 Sept–2 Oct.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Alopecia areata: encouraging response rates with JAK3/TEC inhibition Next Article
Grand debate: Is psoriasis a systemic or skin-only disease? »
« Alopecia areata: encouraging response rates with JAK3/TEC inhibition Next Article
Grand debate: Is psoriasis a systemic or skin-only disease? »
Table of Contents: EADV 2021
Featured articles
Letter from the Editor
Long-term disease control in AD could be in reach with anti-OX40 antibody KHK4083
Late-Breaking News
Targeting OX40 in the treatment of atopic dermatitis meets expectations
Superior EASI scores after switch from dupilumab to upadacitinib
CSU: Novel agent targeting Bruton’s tyrosine kinase leads to disease control
Novel JAK3/TEC blocker leads to maintained re-pigmentation in vitiligo
TYK2 inhibitor deucravacitinib shows impressive long-term response in psoriasis
Tapinarof cream for psoriasis leads to high clearance rates and remittive effect
CSU: Ligelizumab likely safe and effective for adolescents
Long-term disease control in AD could be in reach with anti-OX40 antibody KHK4083
Topical JAK1/JAK2 inhibitor effective in vitiligo
Abrocitinib demonstrates fast itch control and skin clearance in atopic dermatitis
AD patients with stable response fare well with a monthly dose of tralokinumab
Opioid receptor agonist difelikefalin disappoints in AD
Atopic Dermatitis: State of the Art
Upadacitinib beats dupilumab in different body regions
Efficacious 2-year AD control with IL-13 inhibitor tralokinumab
Ruxolitinib cream: a safe treatment for elderly AD patients
Novel and upcoming targeted AD treatment
Psoriasis: What's New?
Existing and upcoming small molecules in psoriasis
Treating psoriasis during pregnancies
A patient-related approach to freedom of disease
Ixekizumab superior to secukinumab in real-world psoriasis study
Nail psoriasis: An important target to be treated
Grand debate: Is psoriasis a systemic or skin-only disease?
Spotlight on Alopecia Areata
JAK1/2: A promising novel treatment target in alopecia areata
Alopecia areata: encouraging response rates with JAK3/TEC inhibition
Related Articles
November 18, 2021
Upadacitinib beats dupilumab in different body regions

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

