The open-label extension ECZTEND trial (NCT03587805) investigated the 2-year efficacy and safety of the anti-IL-13 antibody tralokinumab. Prof. Andrew Blauvelt (Oregon Medical Research Center, OR, USA) presented an interim analysis that included all 345 participants with moderate-to-severe AD who completed 52 weeks on the parent tralokinumab monotherapy trials ECZTRA 1 and 2 (NCT03131648 and NCT03160885) and ≥60 weeks in ECZTEND until the end of April 2020 [1].
The 3 different cohorts in the study consisted of (1) those on continuous treatment who were off tralokinumab for ≤5 weeks between the parent trials (continuous), (2) those with a 6–15 weeks pause of tralokinumab (intermittent), and (3) those with >15 weeks off the drug (washout). The baseline characteristics included a median age of 42 years and 51.2% of women. As the participants had previously been on treatment in ECZTRA 1 and 2, the median baseline EASI scores were relatively low with 2.8 (continuous), 5.8 (intermittent), and 7.6 (washout).
Over 2 years of therapy with tralokinumab, most study participants achieved median EASI improvements in over 90%: 92.7% in the continuous group, 91.7% in the intermittent group, and 92.7% in the washout group(see Figure). Regarding worst weekly pruritus on a numeric rating scale (NRS), continuous treatment led to a median score around 3 (i.e. mild) at the study end. Likewise, median worst eczema-related sleep disturbance was also reduced to a mild degree.
Figure: Median EASI with tralokinumab in parent trials and ECZTEND over 2 years of tralokinumab treatment [1]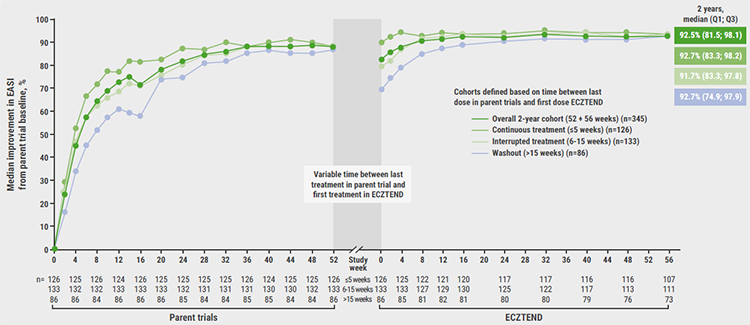
In the 2-year overall cohort, serious adverse events occurred in 4.9% of the participants, with 2% discontinuing due to adverse events. Viral upper respiratory tract infections were most common.
“Over 2 years, tralokinumab provided long-term control of the extent and severity of AD as well as improved itch and reduced sleep interference,” concluded Prof. Blauvelt.
- Blauvelt A. Two-year maintenance of response with tralokinumab in moderate-to-severe atopic dermatitis: Interim analysis of the ECZTEND open-label extension trial. FC01.04, EADV Congress 2021, 29 Sept–2 Oct.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Ruxolitinib cream: a safe treatment for elderly AD patients Next Article
Upadacitinib beats dupilumab in different body regions »
« Ruxolitinib cream: a safe treatment for elderly AD patients Next Article
Upadacitinib beats dupilumab in different body regions »
Table of Contents: EADV 2021
Featured articles
Letter from the Editor
Long-term disease control in AD could be in reach with anti-OX40 antibody KHK4083
Late-Breaking News
Targeting OX40 in the treatment of atopic dermatitis meets expectations
Superior EASI scores after switch from dupilumab to upadacitinib
CSU: Novel agent targeting Bruton’s tyrosine kinase leads to disease control
Novel JAK3/TEC blocker leads to maintained re-pigmentation in vitiligo
TYK2 inhibitor deucravacitinib shows impressive long-term response in psoriasis
Tapinarof cream for psoriasis leads to high clearance rates and remittive effect
CSU: Ligelizumab likely safe and effective for adolescents
Long-term disease control in AD could be in reach with anti-OX40 antibody KHK4083
Topical JAK1/JAK2 inhibitor effective in vitiligo
Abrocitinib demonstrates fast itch control and skin clearance in atopic dermatitis
AD patients with stable response fare well with a monthly dose of tralokinumab
Opioid receptor agonist difelikefalin disappoints in AD
Atopic Dermatitis: State of the Art
Upadacitinib beats dupilumab in different body regions
Efficacious 2-year AD control with IL-13 inhibitor tralokinumab
Ruxolitinib cream: a safe treatment for elderly AD patients
Novel and upcoming targeted AD treatment
Psoriasis: What's New?
Existing and upcoming small molecules in psoriasis
Treating psoriasis during pregnancies
A patient-related approach to freedom of disease
Ixekizumab superior to secukinumab in real-world psoriasis study
Nail psoriasis: An important target to be treated
Grand debate: Is psoriasis a systemic or skin-only disease?
Spotlight on Alopecia Areata
JAK1/2: A promising novel treatment target in alopecia areata
Alopecia areata: encouraging response rates with JAK3/TEC inhibition
Related Articles

November 17, 2021
Opioid receptor agonist difelikefalin disappoints in AD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

