https://doi.org/10.55788/7ad367aa
Ideally, detection of worsening HF in an early phase would allow physicians to intervene timely and proactively to prevent HF-related hospitalisations. Remote monitoring of pulmonary artery pressures (PAP) has emerged as a valuable technique for ambulatory haemodynamic monitoring in HF patients [1]. PAP is a marker of haemodynamic congestion, which occurs several weeks before symptoms develop, providing a possibility for early intervention. The multicentre, clinical, open-label, randomised MONITOR-HF (NTR7672) study evaluated whether assessing haemodynamic congestion based on filling pressures instead of clinical congestion can further improve patients’ quality of life (QoL) and clinical outcomes [2,3].
The study enrolled 348 participants with chronic HF defined as NYHA class III who had at least 1 HF hospitalisation in the Netherlands in the previous 12 months. They were randomised (1:1) either to standard-of-care or pulmonary artery-guided therapy. The latter group received a small, wireless sensor implanted into the pulmonary artery via the femoral vein. All participants had a mean age of 69 and an “appropriate background therapy,” according to Prof. Jasper Brugts (Erasmus University Medical Centre, the Netherlands). Their mean ejection fraction was 30%. The primary endpoint of this open-label trial was quality of life, assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) at 12 months. Total HF hospitalisations were assessed as a secondary outcome.
At 12 months, the average change in the KCCQ overall summary score improved by +7 points in the monitoring group and -0.2 points in the usual care group, yielding a mean difference between groups of 7.1 points in favour of monitoring (P=0.013). This difference persisted during the follow-up period of 1.8 years. During this time, 117 HF hospitalisations or urgent visits occurred in the monitoring group compared with 212 in the usual care group. “This is a meaningful difference and represents a 44% reduction in HF hospitalisation,” Prof. Brugts emphasised (HR 0.56; 95% CI 0.38–0.84; P<0.01; see Figure).
Figure: Reduction in the number of total HF hospitalisations in the MONITOR-HF trial [2]
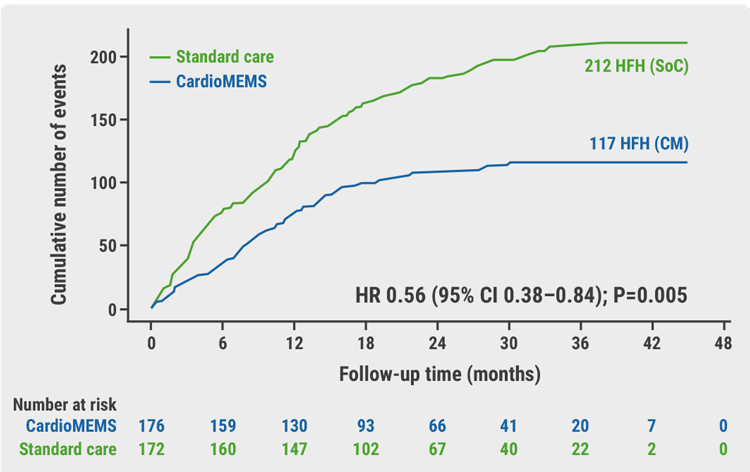
Cox regression analysis Andersen-Gill method (recurring events). CI, confidence interval; CM, CardioMems heart failure system; HFH, heart failure hospitalisations; HR, hazard ratio; SoC, standard of care.
Subgroup analyses showed that this treatment benefit was consistent in subgroups with an ejection fraction of ≤40% and >40%. E.g. in patients with an ejection fraction of ≤40% the event rate/patient-year in the intervention group was 0.35 versus 0.68 in the standard of care group (HR =.53 (95% CI 0.31-0.88). Moreover, In in the intervention group, a significant reduction in NT-proBNP was seen (between-group-difference −669 pg/ml; P = 0.013).
Prof. Brugts explained that the positive effect is induced primarily by changes in diuretics. Diuretics could be optimised based on PAP as a surrogate of left ventricular filling pressures. Therefore, participants in the intervention group have been in a chronically better decongestive state. Furthermore, the implant technology showed to be safe and reliable.
- Abraham WT, et al. The Lancet. 2011;377(9766):658–666.
- Brugts JJ. Remote hemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): A randomised controlled clinical trial. Session Late breaking clinical trials: Chronic HF and cardiomyopathies, Heart Failure 2023, 20–23 May, Prague, Czechia.
- Brugts JJ, et al.Lancet 2023;May 20. DOI:10.1016/S0140-6736(23)00923-6.
Posted on
Previous Article
« TRACER-HF: Trientine reduced biomarkers up to 8 weeks Next Article
Sacubitril/valsartan reduces natriuretic peptides in HF patients with ejection fraction >40% »
« TRACER-HF: Trientine reduced biomarkers up to 8 weeks Next Article
Sacubitril/valsartan reduces natriuretic peptides in HF patients with ejection fraction >40% »
Table of Contents: HFA 2023
Featured articles
Chronic Heart Failure — What You Need to Know
Sacubitril/valsartan reduces natriuretic peptides in HF patients with ejection fraction >40%
Clinically relevant reduction in HF hospitalisation due to haemodynamic monitoring
TRACER-HF: Trientine reduced biomarkers up to 8 weeks
Dapagliflozin improves LAVI, LV mass, and concentration of natriuretic peptides after 6 months
NUDGE-FLU: Repeated electronic nudges improve flu vaccination rates in patients with HF
Novel Therapeutics in Cardiomyopathy
Patisiran benefits maintained over 18 months in patients with transthyretin amyloidosis
Aficamten may lower symptom burden in non-obstructive hypertrophic cardiomyopathy
What is New in Acute Heart Failure?
Standardised diuretic protocol significantly increases natriuresis in acute HF
Low concentrations of VEGF-C: a negative prognostic factor
Prevention and Comorbid Conditions of Heart Failure
VOICE-COVID-II : Alexa successful in SARS-CoV-2 symptoms screening
HF patients with metabolic dysfunction at high risk to develop depressive symptoms
Best of the Posters
Frequent co-existence of atrial fibrillation and obstructive sleep apnoea in stroke patients
Protein-bound uremic toxins predict HF events and death in patients with CKD
Related Articles
September 23, 2020
Ertugliflozin passes post-approval cardiovascular safety test
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

