https://doi.org/10.55788/49bc6af4
Treatment options for HCM have been limited and were predominantly directed towards symptom control obstruction [1]. Aficamten is a next-in-class cardiac myosin inhibitor designed to target the myocardial hypercontractility and impaired relaxation that is responsible for the pathophysiology of non-obstructive HCM, resulting in the generation of symptoms and function impairment. This was the rationale to explore aficamten in Cohort 4 of the REDWOOD-HCM trial (NCT04219826) as a potential novel therapy for non-obstructive HCM. Prof. Ahmad Masri (Oregon Health & Science University, OR, USA) presented the results [2].
In Cohort 4, 41 patients with symptomatic non-obstructive HCM (NYHA class II/III) with a left ventricular ejection fraction (LVEF) of ≥60% and NT-proBNP concentrations of >300 pg/mL were screened and treated with aficamten in addition to standard-of-care. Aficamten in available doses of 5, 10 and 15 mg was up-titrated if LVEF was ≥55%, maintained if LVEF was 50–54%, down-titrated if LVEF was <50%, and discontinued in case of LVEF <40%.
At 10 weeks, dose titration of the myosin inhibitor led to a modest and reversible reduction in LVEF from baseline of 5.5%. No treatment interruptions or down-titration events according to the protocol were necessary. The participants showed a mean improvement in the Kansas City Cardiomyopathy Questionnaire of 10.6 points (P<0.0001 for change from baseline to 10 weeks). Also, 58% of participants had a clinical reduction in symptom burden, with almost half of them reporting moderate to very large improvements (see Figure). “This far exceeds what you expect from placebo,” Prof. Masri said. Moreover, 56% of all participants demonstrated function improvement of ≥1 NYHA class, and 28% of participants achieved NYHA class I and became asymptomatic by week 10. In addition, the frequency of angina was reduced from daily or weekly to weekly or monthly upon treatment.
Figure: Improvement in the Kansas City Cardiomyopathy Questionnaire following aficamten therapy [2]
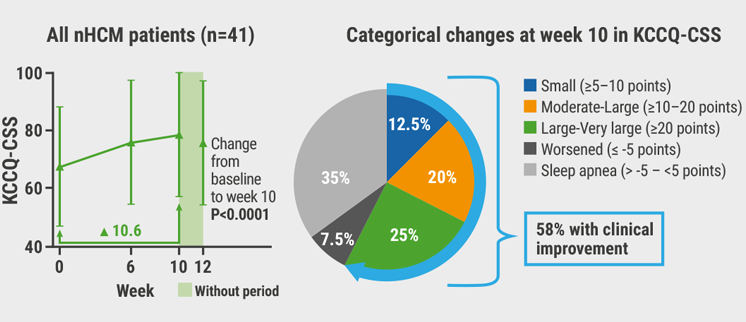
nHCM, non-obstructive hypertrophic cardiomyopathy; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire - Clinical Summary Score.
The improvement of symptoms was accompanied by distinct reductions in cardiac biomarkers. Significant improvement in NT-proBNP was seen with an average decrease of 66% (P<0.0001). At 10 weeks, 3 (7.3%) participants experienced LVEF <50%, but all LVEF returned to baseline levels after 2 weeks of washout.
In addition, there were no serious adverse events attributed to aficamten therapy. A subgroup analysis showed a consistent treatment effect across multiple subgroups independent of responder definition. Notably, 7 patients with mid-cavitary obstruction, who are often excluded from other studies but suffer substantially from limiting symptoms, also showed reductions in both biomarkers and symptom improvement. After these promising results, a phase 3 study is planned to further evaluate aficamten in non-obstructive HCM.
- Packard E, et al. Cardiol Ther 2022:11:491-507.
- Masri A. REDWOOD-HCM-Cohort 4: Aficamten in non-obstructive HCM. Session Late breaking clinical trials: Chronic HF and cardiomyopathies, Heart Failure 2023, 20–23 May 2023, Prague, Czechia.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Sacubitril/valsartan reduces natriuretic peptides in HF patients with ejection fraction >40% Next Article
Patisiran benefits maintained over 18 months in patients with transthyretin amyloidosis »
« Sacubitril/valsartan reduces natriuretic peptides in HF patients with ejection fraction >40% Next Article
Patisiran benefits maintained over 18 months in patients with transthyretin amyloidosis »
Table of Contents: HFA 2023
Featured articles
Chronic Heart Failure — What You Need to Know
Sacubitril/valsartan reduces natriuretic peptides in HF patients with ejection fraction >40%
Clinically relevant reduction in HF hospitalisation due to haemodynamic monitoring
TRACER-HF: Trientine reduced biomarkers up to 8 weeks
Dapagliflozin improves LAVI, LV mass, and concentration of natriuretic peptides after 6 months
NUDGE-FLU: Repeated electronic nudges improve flu vaccination rates in patients with HF
Novel Therapeutics in Cardiomyopathy
Patisiran benefits maintained over 18 months in patients with transthyretin amyloidosis
Aficamten may lower symptom burden in non-obstructive hypertrophic cardiomyopathy
What is New in Acute Heart Failure?
Standardised diuretic protocol significantly increases natriuresis in acute HF
Low concentrations of VEGF-C: a negative prognostic factor
Prevention and Comorbid Conditions of Heart Failure
VOICE-COVID-II : Alexa successful in SARS-CoV-2 symptoms screening
HF patients with metabolic dysfunction at high risk to develop depressive symptoms
Best of the Posters
Frequent co-existence of atrial fibrillation and obstructive sleep apnoea in stroke patients
Protein-bound uremic toxins predict HF events and death in patients with CKD
Related Articles
February 26, 2020
ISCHEMIA trial: Invasive treatment only better for angina burden
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

