Lina
El Murr
× Lina
El Murr
* First author
Affiliation
Gustave Roussy, Université Paris-Saclay, Département d’Hématologie, Villejuif, F-94805, France.
* First author
Affiliation
Gustave Roussy, Université Paris-Saclay, Département d’Hématologie, Villejuif, F-94805, France.
Jean Baptiste
Micol
(email)× Jean Baptiste
Micol
(orcid) (email)
Affiliation
Gustave Roussy, Université Paris-Saclay, Département d’Hématologie, Villejuif, F-94805, France.
Affiliation
Gustave Roussy, Université Paris-Saclay, Département d’Hématologie, Villejuif, F-94805, France.
https://doi.org/10.55788/fbbf131a
INTRODUCTION
The understanding of acute myeloid leukaemia (AML) has evolved drastically over the past decade. With the lens being shifted to a molecular level, it has unveiled a large heterogenous spectrum of molecular identities that define the disease, its evolution and prognosis, and guide the treatment strategies.1 However, AML remains very challenging to treat in high-risk elderly patients, especially TP53 AML patients, unfit for intensive chemotherapy.
Ten years ago, the AZA-AML-001 trial compared 5’-Azacitidine to the conventional care regimens (CCR; including best supportive care, subcutaneous cytarabine or intensive chemotherapy) in treating older patients (65 years or older) newly diagnosed AML with more than 30% of bone marrow (BM) blasts.2 Patients eligible for hematopoietic stem cell transplantation were not included in this trial. The trial failed to show a significant improvement in the overall survival (OS) in the global population but was considered safe and manageable in this difficult-to-treat AML population. Moreover, in a subgroup analysis of the trial, 5’-Azacitidine was shown to be more beneficial in AML patients whose cytogenetics inflicted a poor prognosis (such as chromosome 5 or 7 abnormalities or complex karyotypes) or whose molecular profiles harboured an unfavourable mutation. TP53-mutated AML patients had an improved median OS compared to their counterparts in the CCR arm with a median OS of 7.2 vs 2.4 months.3 The potential increased sensitivity of TP53-mutated AML to hypomethylating agent was confirmed by the high rate of complete response (CR) in TP53-mutated AML patients treated with Decitabine even if these responses were not long-lasting,4 suggesting a potential interest in hypomethylating agent-based combination therapies.
The addition of Venetoclax to 5’-Azacitidine led to improved survival compared with 5’-Azacitidine alone in patients with newly diagnosed AML unfit for intensive treatment (VIALE-A trial).5 However, these findings were not the same in all the population subgroups. Individuals with unfavourable molecular profiles and more specifically, those carrying the TP53 mutation, had a lower benefit from the combination of 5’-Azacitidine -Venetoclax than individuals with more favourable profiles,6 when compared to 5’-Azacitidine alone Moreover, in pooled data from the phase 1b trial (NCT02203773) and the phase 3 trial (VIALE-A), the median OS for TP53-mutated patients, TP53-WT with FLT3-ITD-mutated or K-NRAS-mutated patients and TP53-WT with FLT3-ITD-WT or K-NRAS-WT were 5.52 months (95%CI 2.19-7.59), 12.12 months (95%CI 7.26-15.15), and 26.51 months (95%CI 20.24-32.69) respectively. These data were confirmed by real-life studies showing a very poor OS for TP53-mutated AML patients.7
Other combinations failed to improve survival compared to 5’-Azacitidine alone or CCR; for instance, the ENHANCE-2 trial failed to prove that the monoclonal antibody (anti-CD47) Magrolimab would have a survival benefit when added to 5’-Azacitidine, similarly to Eprenetapopt (TP53-reactivating compound) which did not have an added benefit when given with 5’-Azacitidine in treating TP53-mutated AML patients.
Despite the poor results, responses to treatment have been achieved in a few cases. The following three case reports may not be representative of the whole unfit TP53 AML population but will shed light on the molecular complexity of this subgroup of TP53-mutated AML and its potential therapeutic response.
CASE 1
A 75-year-old female patient, with an ECOG performance status of 2 to 3, known to have Chronic Obstructive Pulmonary Disease and Aortic insufficiency, was diagnosed with AML with complex karyotype and TP53 mutation.
The diagnosis was based on a complete blood count at presentation that revealed a white blood cell count (WBC) of 3.5 G/L with 5% blasts, haemoglobin of 9.9 g/dl and platelets count of 95 G/L. A bone marrow aspirate was performed revealing AML with myelodysplasia-related changes (22% of blasts) (fig 1.A) with a Leukemia-associated immunophenotyping (LAIP) on flow cytometry with positive CD34/33/117/7 (fig 1.B). Cytogenetic analysis revealed a complex karyotype including a deletion of chromosome 17 (fig 1.C). An NGS revealed a TP53 H179N mutation with a 65% variant allele frequency (VAF). The multidisciplinary tumour board was held and treatment with 5’-Azacitidine + Venetoclax was decided. Evaluation after three cycles of 5’-Azacitidine -Venetoclax was in favour of a complete cytologic remission (fig 1.D), a negative minimal residual disease (MRD) on flow cytometry (fig 1.E), and a cytogenetic remission (fig 1.F). However, no NGS was done.
This is one of the double-hit TP53-mutated AML patients who presented the best response to 5’-Azacitidine-Venetoclax in our centre. We picked this example as this patient reached a complete cytological, immunophenotypic, and cytogenetic response with 5’-Azacitidine-Venetoclax, however, this patient had a relapse of his AML after the 10th cycle and died shortly after.
This case translates the idea that a complete remission (CR) does not necessarily dictate a better overall survival. A study has shown the efficacy of a 10-day treatment with Decitabine in high-risk TP53-mutated AML patients, in achieving complete molecular remission. However, this high degree of decitabine sensitivity allowed outgrowth of a preexisting subclone in all cases, implicating an early relapse.4 This was also shown in the analysis of the phase III study VIALE-A trial and a preceding phase IB study that showed a high CR rate in TP53-mutated AML when treated with the combination of 5’-Azacitidine-Venetoclax without improving the overall survival or affecting the duration of remission.8
Fig 1. Bone marrow evaluation of a TP53-mutated AML patient at diagnosis (A-C) and after three cycles of 5’-Azacitidine-Venetoclax (D-F).

Myeloid blasts shown on the bone marrow aspiration evaluation (A), confirmed on the flow cytometry with positive CD34/33/117/7 (B) and associated with a complex karyotype including a deletion of chromosome 17 (C), at diagnosis. After 3 treatment cycles with 5’-Azacitidine-Venetoclax, evaluation revealed a complete remission with no or minimal blasts on the bone marrow analysis (D), confirmed by the flow cytometry with a low CD34/33/117/7 signalling (E) and a karyotype no longer expressing the chromosome 17 deletion (F). CASE 2
An 86-year-old female patient, with an ECOG performance status of 1, with a known medical history of breast cancer treated with partial mastectomy and axillary lymph node dissection and radiotherapy, in remission since 2016. She was diagnosed with t-AML in June 2020 based on a bone marrow aspiration revealing 28% blasts with LAIP on flow cytometry, a normal karyotype, and with an NGS revealing a DNMT3A, IDH2, TET2, SH2B3, and TP53 mutations [DNMT3A R730H & A639V (VAF 27% and 14%), IDH2 R140Q (VAF 12%), TET2 R123C & N275Ifs (VAF 3% and 3%), SH2B3 splice exon2 (VAF 3%), TP53 R248Q & C124F (VAF 2% and 2%)]. As expected, the choice of treatment which the multidisciplinary tumour board agreed on was 5’-Azacitidine-Venetoclax and an evaluation after the second cycle was in favour of complete cytologic remission with an NGS panel expressing the DNMT3A, IDH2, TET2, and SH2B3 mutations without the TP53 mutation. In October 2023, after 43 cycles of 5’-Azacitidine-Venetoclax, the patient is still in complete response.
This case highlights the importance of the risk-stratification of the AML based on VAF of the TP53 mutation and not by the sole presence or absence of this culprit mutation. For instance, it has been suggested that the International Consensus Classification (ICC) may have under-estimated the toll that has a ‘multi-hit’ TP53 mutation with a VAF of <10% on the prognosis, in the same way that the World Health Organization (WHO) may under-estimated the poor prognosis inflicted by the presence of a monoallelic TP53 MDS with 10-19% blasts as well as TP53mut VAF ≤49% in the presence of CK.4,8 These two classifications wouldn’t classify patients with TP53 mutation of VAF < 10% as high-risk AML which seems to be the case for our patient in Case 2.
CASE 3
A 66-year-old female, with an ECOG performance status of 1, was known to have breast cancer diagnosed in 2011 with metastases to the bones despite five lines of treatment. In September 2020, she initially presented with a myelodysplastic syndrome with an excess of blasts; MDS EB-1 with a very high R-IPSSM risk score, a complex karyotype, and an NGS panel revealing a TP53 splice exon 4 (11%) & R273H (7%). This patient was transfusion-dependent with an initial hemoglobin level of 7.6 g/dl.
The multidisciplinary tumour board was held and decided to treat with 5’-Azacitidine. An evaluation after a third cycle was in favour of a progression into AML with 35% blasts, with worsening of the transfusion dependence (haemoglobin of 6.6g/dl and platelets of 12 G/L). The NGS panel revealed then mutations of TP53 R273H (21%) and IDH1 R132C (23%). A treatment with Ivosidenib monotherapy was therefore initiated and 3 months into therapy, the patient became transfusion-independent with a partial response (fig 2A and 2B) and neutrophil recovery. Unfortunately, the patient died 9 months after initiation of Ivosidenib treatment from a progression of breast cancer with a stable AML disease.
Fig 2. Immunophenotypic evaluation by flow cytometry of the bone marrow of the patient before (A) and 3 months after treatment with Ivosidenib (B), showing the shift in signalling and the net decrease in the blasts’ expression and differentiation syndrome.
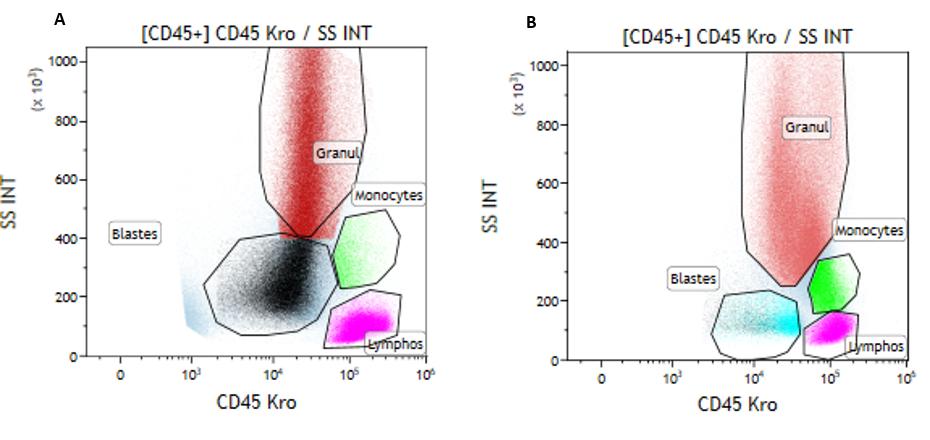
This case highlights the potential benefit of targeting mutations or pathways other than the TP53, such as the above-mentioned IDH1 mutation, in a relapsed or refractory AML. It also suggests that in case of a relapsed or refractory disease, a new NGS evaluation could be of clinical significance as some subclones could emerge and proliferate while targeting the initial mutation. This key concept was highlighted in the IDHENTIFY trial which showed an improved response to treatment with enasidenib compared to CCR in late-stage IDH2-mutated relapsed or refractory acute myeloid leukaemia where around 14% of the patients had an associated TP53 mutation.11,12 Moreover, a recent abstract described, through single-cell DNA profiling, the acquisition of an IDH1 mutation in a TP53 AML patient treated with CD47 antibody, 5’-Azacitidine and Venetoclax, thus confirming that this phenomenon may not be incidental.13CONCLUSIONS
Here, we deliberately chose unfit TP53 AML patients who had the best response to the treatment in our centre. Unfortunately, especially in the case of multi-hit TP53 mutations, the disease is considered as not curable whatever the intensity of the treatment. When possible it is recommended to enroll these patients into clinical trials for alternative strategies. So far “promising drugs” have not translated into “efficient drugs” but the substantial number of abstracts with new drugs tested in TP53 AML this year at the ASH congress is encouraging and highlights that emerging therapies seek to address these unmet needs.
New therapeutic strategies may emerge in the future. Indeed, the availability of NGS technologies represents a breakthrough in the field of AML, especially for a better understanding of the disease pathogenesis. TP53 mutations are known to be enriched in therapy-related myeloid neoplasm14 and the recent identification of clonal hematopoiesis (CH) as a preleukemic state and its high incidence in cancer survivors may open up new perspectives. Incidental detection of CH through liquid profiling of cell-free DNA, germline testing or exploration of cytopenia became a custom in clinical practice. It helps to identify patients at high risk of developing AML or MDS, especially in the case of TP53 CH, and contributes to the development of prevention strategies.
More and more centres created CH clinic or specific molecular tumour boards to improve clinical management of patients15 and some clinical trials16 are now opening for early therapeutic interventions which could lead the way to new perspectives in the management of patients at risk of developing TP53 AML.
CONFLICT OF INTEREST
A 75-year-old female patient, with an ECOG performance status of 2 to 3, known to have Chronic Obstructive Pulmonary Disease and Aortic insufficiency, was diagnosed with AML with complex karyotype and TP53 mutation.
The diagnosis was based on a complete blood count at presentation that revealed a white blood cell count (WBC) of 3.5 G/L with 5% blasts, haemoglobin of 9.9 g/dl and platelets count of 95 G/L. A bone marrow aspirate was performed revealing AML with myelodysplasia-related changes (22% of blasts) (fig 1.A) with a Leukemia-associated immunophenotyping (LAIP) on flow cytometry with positive CD34/33/117/7 (fig 1.B). Cytogenetic analysis revealed a complex karyotype including a deletion of chromosome 17 (fig 1.C). An NGS revealed a TP53 H179N mutation with a 65% variant allele frequency (VAF). The multidisciplinary tumour board was held and treatment with 5’-Azacitidine + Venetoclax was decided. Evaluation after three cycles of 5’-Azacitidine -Venetoclax was in favour of a complete cytologic remission (fig 1.D), a negative minimal residual disease (MRD) on flow cytometry (fig 1.E), and a cytogenetic remission (fig 1.F). However, no NGS was done.
This is one of the double-hit TP53-mutated AML patients who presented the best response to 5’-Azacitidine-Venetoclax in our centre. We picked this example as this patient reached a complete cytological, immunophenotypic, and cytogenetic response with 5’-Azacitidine-Venetoclax, however, this patient had a relapse of his AML after the 10th cycle and died shortly after.
This case translates the idea that a complete remission (CR) does not necessarily dictate a better overall survival. A study has shown the efficacy of a 10-day treatment with Decitabine in high-risk TP53-mutated AML patients, in achieving complete molecular remission. However, this high degree of decitabine sensitivity allowed outgrowth of a preexisting subclone in all cases, implicating an early relapse.4 This was also shown in the analysis of the phase III study VIALE-A trial and a preceding phase IB study that showed a high CR rate in TP53-mutated AML when treated with the combination of 5’-Azacitidine-Venetoclax without improving the overall survival or affecting the duration of remission.8
Fig 1. Bone marrow evaluation of a TP53-mutated AML patient at diagnosis (A-C) and after three cycles of 5’-Azacitidine-Venetoclax (D-F).

Myeloid blasts shown on the bone marrow aspiration evaluation (A), confirmed on the flow cytometry with positive CD34/33/117/7 (B) and associated with a complex karyotype including a deletion of chromosome 17 (C), at diagnosis. After 3 treatment cycles with 5’-Azacitidine-Venetoclax, evaluation revealed a complete remission with no or minimal blasts on the bone marrow analysis (D), confirmed by the flow cytometry with a low CD34/33/117/7 signalling (E) and a karyotype no longer expressing the chromosome 17 deletion (F).
CASE 2
An 86-year-old female patient, with an ECOG performance status of 1, with a known medical history of breast cancer treated with partial mastectomy and axillary lymph node dissection and radiotherapy, in remission since 2016. She was diagnosed with t-AML in June 2020 based on a bone marrow aspiration revealing 28% blasts with LAIP on flow cytometry, a normal karyotype, and with an NGS revealing a DNMT3A, IDH2, TET2, SH2B3, and TP53 mutations [DNMT3A R730H & A639V (VAF 27% and 14%), IDH2 R140Q (VAF 12%), TET2 R123C & N275Ifs (VAF 3% and 3%), SH2B3 splice exon2 (VAF 3%), TP53 R248Q & C124F (VAF 2% and 2%)]. As expected, the choice of treatment which the multidisciplinary tumour board agreed on was 5’-Azacitidine-Venetoclax and an evaluation after the second cycle was in favour of complete cytologic remission with an NGS panel expressing the DNMT3A, IDH2, TET2, and SH2B3 mutations without the TP53 mutation. In October 2023, after 43 cycles of 5’-Azacitidine-Venetoclax, the patient is still in complete response.
This case highlights the importance of the risk-stratification of the AML based on VAF of the TP53 mutation and not by the sole presence or absence of this culprit mutation. For instance, it has been suggested that the International Consensus Classification (ICC) may have under-estimated the toll that has a ‘multi-hit’ TP53 mutation with a VAF of <10% on the prognosis, in the same way that the World Health Organization (WHO) may under-estimated the poor prognosis inflicted by the presence of a monoallelic TP53 MDS with 10-19% blasts as well as TP53mut VAF ≤49% in the presence of CK.4,8 These two classifications wouldn’t classify patients with TP53 mutation of VAF < 10% as high-risk AML which seems to be the case for our patient in Case 2.
CASE 3
A 66-year-old female, with an ECOG performance status of 1, was known to have breast cancer diagnosed in 2011 with metastases to the bones despite five lines of treatment. In September 2020, she initially presented with a myelodysplastic syndrome with an excess of blasts; MDS EB-1 with a very high R-IPSSM risk score, a complex karyotype, and an NGS panel revealing a TP53 splice exon 4 (11%) & R273H (7%). This patient was transfusion-dependent with an initial hemoglobin level of 7.6 g/dl.
The multidisciplinary tumour board was held and decided to treat with 5’-Azacitidine. An evaluation after a third cycle was in favour of a progression into AML with 35% blasts, with worsening of the transfusion dependence (haemoglobin of 6.6g/dl and platelets of 12 G/L). The NGS panel revealed then mutations of TP53 R273H (21%) and IDH1 R132C (23%). A treatment with Ivosidenib monotherapy was therefore initiated and 3 months into therapy, the patient became transfusion-independent with a partial response (fig 2A and 2B) and neutrophil recovery. Unfortunately, the patient died 9 months after initiation of Ivosidenib treatment from a progression of breast cancer with a stable AML disease.
Fig 2. Immunophenotypic evaluation by flow cytometry of the bone marrow of the patient before (A) and 3 months after treatment with Ivosidenib (B), showing the shift in signalling and the net decrease in the blasts’ expression and differentiation syndrome.

This case highlights the potential benefit of targeting mutations or pathways other than the TP53, such as the above-mentioned IDH1 mutation, in a relapsed or refractory AML. It also suggests that in case of a relapsed or refractory disease, a new NGS evaluation could be of clinical significance as some subclones could emerge and proliferate while targeting the initial mutation. This key concept was highlighted in the IDHENTIFY trial which showed an improved response to treatment with enasidenib compared to CCR in late-stage IDH2-mutated relapsed or refractory acute myeloid leukaemia where around 14% of the patients had an associated TP53 mutation.11,12 Moreover, a recent abstract described, through single-cell DNA profiling, the acquisition of an IDH1 mutation in a TP53 AML patient treated with CD47 antibody, 5’-Azacitidine and Venetoclax, thus confirming that this phenomenon may not be incidental.13CONCLUSIONS
Here, we deliberately chose unfit TP53 AML patients who had the best response to the treatment in our centre. Unfortunately, especially in the case of multi-hit TP53 mutations, the disease is considered as not curable whatever the intensity of the treatment. When possible it is recommended to enroll these patients into clinical trials for alternative strategies. So far “promising drugs” have not translated into “efficient drugs” but the substantial number of abstracts with new drugs tested in TP53 AML this year at the ASH congress is encouraging and highlights that emerging therapies seek to address these unmet needs.
New therapeutic strategies may emerge in the future. Indeed, the availability of NGS technologies represents a breakthrough in the field of AML, especially for a better understanding of the disease pathogenesis. TP53 mutations are known to be enriched in therapy-related myeloid neoplasm14 and the recent identification of clonal hematopoiesis (CH) as a preleukemic state and its high incidence in cancer survivors may open up new perspectives. Incidental detection of CH through liquid profiling of cell-free DNA, germline testing or exploration of cytopenia became a custom in clinical practice. It helps to identify patients at high risk of developing AML or MDS, especially in the case of TP53 CH, and contributes to the development of prevention strategies.
More and more centres created CH clinic or specific molecular tumour boards to improve clinical management of patients15 and some clinical trials16 are now opening for early therapeutic interventions which could lead the way to new perspectives in the management of patients at risk of developing TP53 AML.
CONFLICT OF INTEREST
A 66-year-old female, with an ECOG performance status of 1, was known to have breast cancer diagnosed in 2011 with metastases to the bones despite five lines of treatment. In September 2020, she initially presented with a myelodysplastic syndrome with an excess of blasts; MDS EB-1 with a very high R-IPSSM risk score, a complex karyotype, and an NGS panel revealing a TP53 splice exon 4 (11%) & R273H (7%). This patient was transfusion-dependent with an initial hemoglobin level of 7.6 g/dl.
The multidisciplinary tumour board was held and decided to treat with 5’-Azacitidine. An evaluation after a third cycle was in favour of a progression into AML with 35% blasts, with worsening of the transfusion dependence (haemoglobin of 6.6g/dl and platelets of 12 G/L). The NGS panel revealed then mutations of TP53 R273H (21%) and IDH1 R132C (23%). A treatment with Ivosidenib monotherapy was therefore initiated and 3 months into therapy, the patient became transfusion-independent with a partial response (fig 2A and 2B) and neutrophil recovery. Unfortunately, the patient died 9 months after initiation of Ivosidenib treatment from a progression of breast cancer with a stable AML disease.
Fig 2. Immunophenotypic evaluation by flow cytometry of the bone marrow of the patient before (A) and 3 months after treatment with Ivosidenib (B), showing the shift in signalling and the net decrease in the blasts’ expression and differentiation syndrome.

This case highlights the potential benefit of targeting mutations or pathways other than the TP53, such as the above-mentioned IDH1 mutation, in a relapsed or refractory AML. It also suggests that in case of a relapsed or refractory disease, a new NGS evaluation could be of clinical significance as some subclones could emerge and proliferate while targeting the initial mutation. This key concept was highlighted in the IDHENTIFY trial which showed an improved response to treatment with enasidenib compared to CCR in late-stage IDH2-mutated relapsed or refractory acute myeloid leukaemia where around 14% of the patients had an associated TP53 mutation.11,12 Moreover, a recent abstract described, through single-cell DNA profiling, the acquisition of an IDH1 mutation in a TP53 AML patient treated with CD47 antibody, 5’-Azacitidine and Venetoclax, thus confirming that this phenomenon may not be incidental.13
CONCLUSIONS
Here, we deliberately chose unfit TP53 AML patients who had the best response to the treatment in our centre. Unfortunately, especially in the case of multi-hit TP53 mutations, the disease is considered as not curable whatever the intensity of the treatment. When possible it is recommended to enroll these patients into clinical trials for alternative strategies. So far “promising drugs” have not translated into “efficient drugs” but the substantial number of abstracts with new drugs tested in TP53 AML this year at the ASH congress is encouraging and highlights that emerging therapies seek to address these unmet needs.
New therapeutic strategies may emerge in the future. Indeed, the availability of NGS technologies represents a breakthrough in the field of AML, especially for a better understanding of the disease pathogenesis. TP53 mutations are known to be enriched in therapy-related myeloid neoplasm14 and the recent identification of clonal hematopoiesis (CH) as a preleukemic state and its high incidence in cancer survivors may open up new perspectives. Incidental detection of CH through liquid profiling of cell-free DNA, germline testing or exploration of cytopenia became a custom in clinical practice. It helps to identify patients at high risk of developing AML or MDS, especially in the case of TP53 CH, and contributes to the development of prevention strategies.
More and more centres created CH clinic or specific molecular tumour boards to improve clinical management of patients15 and some clinical trials16 are now opening for early therapeutic interventions which could lead the way to new perspectives in the management of patients at risk of developing TP53 AML.
CONFLICT OF INTEREST
Lina El Murr has no conflict of interest to declare. Jean Baptiste Micol: Honoraria - Jazz Pharmaceuticals (less than $10,000 in a single calendar year), AstraZeneca (less than $10,000 in a single calendar year), Astellas Pharma (less than $10,000 in a single calendar year); Consulting or Advisory Role - AbbVie (less than $10,000 in a single calendar year), Gilead Sciences (less than $10,000 in a single calendar year); Travel, Accommodations, Expenses - AbbVie (less than $10,000 in a single calendar year).
ACKNOWLEDGEMENTS
The authors would like to thank the organisers of the 4th European Congress on Controversies in Leukemias.
REFERENCES
- Döhner H, Wei AH, Appelbaum FR, Craddock C, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):345–1377. doi: 10.1182/blood.2022016867
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of 5’-Azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. doi: 10.1182/blood-2015-01-621664
- Döhner H, Dolnik A, Tang L, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with 5’-Azacitidine or conventional care. Leukemia. 2018;32(12):2546-2557. doi: 10.1038/s41375-018-0257-z
- Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023-2036. doi: 10.1056/nejmoa1605949
- DiNardo CD, Jonas BA, Pullarkat V, et al. 5’-Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi: 10.1056/nejmoa2012971
- Döhner H, Pratz KW, DiNardo CD, et al. ELN risk stratification is not predictive of outcomes for treatment-naïve patients with acute myeloid leukemia treated with venetoclax and 5’-Azacitidine. Blood. 2022;140(Supplement 1):1441-1444. doi: 10.1182/blood-2022-169509
- Willekens C, Chraibi S, Decroocq J, et al. Reduced venetoclax exposition to seven days of azacitidine is efficient in treatment-naïve patients with acute myeloid leukemia. Blood. 2022;140(Supplement 1):537–538. doi: 10.1182/blood-2022-165464
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of Venetoclax and 5’-Azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi: 10.1158/1078-0432.ccr-21-3467
- Shah MV, Kutyna M, Shah S, et al. Comparison of World Health Organization and International Consensus Classification guidelines for myeloid neoplasms harboring TP53-mutations using an independent international cohort. Blood. 2023;142(Supplement 1):3243-3243. doi: 10.1182/blood-2023-187860
- Stengel A, Meggendorfer M, Walter W, et al. Interplay of TP53 allelic state, blast count, and complex karyotype on survival of patients with AML and MDS. Blood Adv. 2023;7(18):5540-5548. doi: 10.1182/bloodadvances.2023010312
- De Botton S, Montesinos P, Schuh AC, et al. Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: a randomized phase 3 trial. Blood. 2023;141(2):156-167. doi: 10.1182/blood.2021014901
- De Botton S, Risueño A, Schuh AC, et al. Overall survival by IDH2 mutant allele (R140 or R172) in patients with late-stage mutant-IDH2 relapsed or refractory acute myeloid leukemia treated with enasidenib or conventional care regimens in the phase 3 IDHENTIFY trial. J Clin Oncol. 2022;40(16_suppl):7005–7005. doi: 10.1200/jco.2022.40.16_suppl.7005
- Ayoub E, Li L, Muftuoglu M, et al. Single-cell genomics and proteomics reveals Venetoclax-resistant monocytic differentiation of TP53 LOH clones in TP53 mutant AML. Blood. 2023;142(Supplement 1):5014–5014. doi : 10.1182/blood-2023-187121
- Renneville A, Bernard E, Micol JB. Therapy-related myelodysplastic syndromes in the genomics era. Bulletin Du Cancer. 2023;110(11):1129–1140. doi: 10.1016/j.bulcan.2023.02.022
- Renneville A, Bernard E, Micol JB. Clonal hematopoiesis in patients with cancer: How much should we care? JCO Precis Oncol. 2023;7:e2300417. doi: 10.1200/po.23.00417
- Clinicaltrials.gov. (n.d.). Clinicaltrials.gov. Retrieved February 21, 2024, from https://www.clinicaltrials.gov/study/NCT05641831
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Is allogeneic transplantation necessary in acute myeloid leukaemia of intermediate risk in the first complete remission?
Next Article
Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)? »
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved.
Terms and Conditions
| Privacy Policy
- Döhner H, Wei AH, Appelbaum FR, Craddock C, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):345–1377. doi: 10.1182/blood.2022016867
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of 5’-Azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. doi: 10.1182/blood-2015-01-621664
- Döhner H, Dolnik A, Tang L, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with 5’-Azacitidine or conventional care. Leukemia. 2018;32(12):2546-2557. doi: 10.1038/s41375-018-0257-z
- Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023-2036. doi: 10.1056/nejmoa1605949
- DiNardo CD, Jonas BA, Pullarkat V, et al. 5’-Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi: 10.1056/nejmoa2012971
- Döhner H, Pratz KW, DiNardo CD, et al. ELN risk stratification is not predictive of outcomes for treatment-naïve patients with acute myeloid leukemia treated with venetoclax and 5’-Azacitidine. Blood. 2022;140(Supplement 1):1441-1444. doi: 10.1182/blood-2022-169509
- Willekens C, Chraibi S, Decroocq J, et al. Reduced venetoclax exposition to seven days of azacitidine is efficient in treatment-naïve patients with acute myeloid leukemia. Blood. 2022;140(Supplement 1):537–538. doi: 10.1182/blood-2022-165464
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of Venetoclax and 5’-Azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi: 10.1158/1078-0432.ccr-21-3467
- Shah MV, Kutyna M, Shah S, et al. Comparison of World Health Organization and International Consensus Classification guidelines for myeloid neoplasms harboring TP53-mutations using an independent international cohort. Blood. 2023;142(Supplement 1):3243-3243. doi: 10.1182/blood-2023-187860
- Stengel A, Meggendorfer M, Walter W, et al. Interplay of TP53 allelic state, blast count, and complex karyotype on survival of patients with AML and MDS. Blood Adv. 2023;7(18):5540-5548. doi: 10.1182/bloodadvances.2023010312
- De Botton S, Montesinos P, Schuh AC, et al. Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: a randomized phase 3 trial. Blood. 2023;141(2):156-167. doi: 10.1182/blood.2021014901
- De Botton S, Risueño A, Schuh AC, et al. Overall survival by IDH2 mutant allele (R140 or R172) in patients with late-stage mutant-IDH2 relapsed or refractory acute myeloid leukemia treated with enasidenib or conventional care regimens in the phase 3 IDHENTIFY trial. J Clin Oncol. 2022;40(16_suppl):7005–7005. doi: 10.1200/jco.2022.40.16_suppl.7005
- Ayoub E, Li L, Muftuoglu M, et al. Single-cell genomics and proteomics reveals Venetoclax-resistant monocytic differentiation of TP53 LOH clones in TP53 mutant AML. Blood. 2023;142(Supplement 1):5014–5014. doi : 10.1182/blood-2023-187121
- Renneville A, Bernard E, Micol JB. Therapy-related myelodysplastic syndromes in the genomics era. Bulletin Du Cancer. 2023;110(11):1129–1140. doi: 10.1016/j.bulcan.2023.02.022
- Renneville A, Bernard E, Micol JB. Clonal hematopoiesis in patients with cancer: How much should we care? JCO Precis Oncol. 2023;7:e2300417. doi: 10.1200/po.23.00417
- Clinicaltrials.gov. (n.d.). Clinicaltrials.gov. Retrieved February 21, 2024, from https://www.clinicaltrials.gov/study/NCT05641831
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Is allogeneic transplantation necessary in acute myeloid leukaemia of intermediate risk in the first complete remission? Next Article
Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)? »
« Is allogeneic transplantation necessary in acute myeloid leukaemia of intermediate risk in the first complete remission? Next Article
Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)? »
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

