A recent analysis of data from 78 clinical trials of adalimumab, including 29,987 patients, demonstrated an overall safety profile consistent with previous findings and with the TNFi class in general. No new safety signals or tolerability issues with adalimumab treatment were identified and, for most indications, the mortality rate was below what would be expected in an age-adjusted and sex-adjusted population.
According to Prof. Gerd Burmester (Charité University Hospital, Free University and Humboldt University of Berlin, Germany), aspects of efficacy of adalimumab are well known, but patients now want to be informed about the safety of the treatment, especially as it often involves long-term use.
Adalimumab’s long-term safety was previously reported in 23,458 patients representing up to 12 years of clinical trial exposure in RA, juvenile idiopathic arthritis (JIA), ankylosing spondylitis (AS), PsA, Ps, and CD. Mortality rates of patients under adalimumab were not increased compared with rates expected for the general population.[16] Infections represented the most frequently reported serious AEs across these indications. Burmester et al. reported an updated analysis examining the long-term safety of adalimumab in adult patients with RA, AS, non-radiographic axial SpA (nr-axSpA), peripheral SpA (pSpA), PsA, Ps, hidradenitis suppurativa (HS), CD, UC, and non-infectious uveitis (UV). Safety assessments included all (serious) AEs that occurred after the first adalimumab dose and up to 70 days after the last study dose.
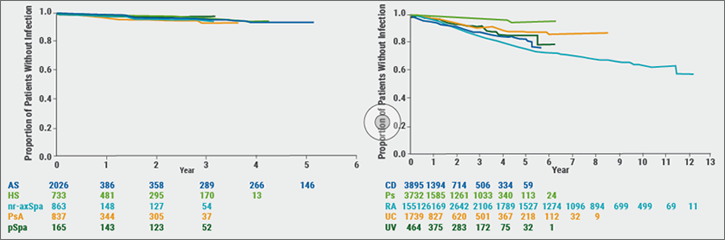
The majority of patients who were exposed to adalimumab were those in RA studies (n=15,512). The most frequently reported serious AE of interest was infection with the highest incidences in CD, RA, UV, and UC. The most commonly reported serious infections were pneumonia (0.6/100 patient-years) and notably cellulitis (0.2/patient-years). The risk of a serious infection event was generally stable over time and for all indications (see Figure 3).[17]
Figure: Time to first serious infection by indication [17]
The assessment of malignancies showed that the overall rate of serious malignancies, excluding lymphoma, hepatosplenic T-cell lymphoma, leukaemia, non-melanoma skin cancer (NMSC), and melanoma was 0.6/100 patient-years. The highest rates of malignancies were seen among RA, UV, and UC studies suggesting the role of the underlying disease. For NMSC, the overall rate was 0.1/100 patient-years.
The time to first malignancy, with the exclusion of the aforementioned exceptions, did not show any marked difference between indications. From the assessment of standardised mortality rates emerged that overall and for most of the adalimumab populations (i.e. AS, PsA, Ps, UC, CD, and RA), the observed number of deaths was below what would be expected in an age-adjusted and sex-adjusted population. For HS, nr-axSpA, pSpA, and UV studies, the small size of these trials precluded accurate assessment of the SMR, and the 95% CIs all included 1.0. Prof. Burmester concluded that the efficacy and safety data continue to support the well-established benefits of adalimumab for the approved indications.[17]
- Burmester G, et al. Ann Rheum Dis. 2013;72(4):517-24.
- Burmester G, et al. Abstract OP0233. EULAR 2018.
Posted on
Previous Article
« Letter from the Editor Next Article
Biologic drug levels and infection risk, and clinical effectiveness of sarilumab »
« Letter from the Editor Next Article
Biologic drug levels and infection risk, and clinical effectiveness of sarilumab »
Table of Contents: EULAR 2018
Featured articles
Rheumatoid Arthritis
Switching to biosimilar bDMARDs is safe and efficacious
No significant differences when tapering TNF blockers versus csDMARDs
Confirmation of long-term safety profile adalimumab across indications
Ankylosing Spondylitis
Clinical effect of vedolizumab on articular manifestations spondyloarthritis associated with IBD
Synergistic effect NSAIDs plus TNFi in slowing radiographic progression in ankylosing spondylitis patients
Osteoporosis and Osteoarthritis
Systemic Sclerosis and Systemic Lupus Erythematosus
Promising results rituximab in systemic sclerosis, and systemic lupus erythematosus classification criteria
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com