Paccou et al. evaluated baseline characteristics of CD and UC patients treated with vedolizumab, as well as assessing the effect of vedolizumab on joint manifestations in patients with inflammatory bowel disease (IBD)-associated SpA, and new onset of SpA under vedolizumab.
This study was performed as a single-centre, retrospective, and observational study, conducted between July 2014 and July 2017. It involved reviewing the charts of all patients with IBD who had undergone treatment with vedolizumab for more than 3 months. A total of 171 patients diagnosed with IBD were treated with vedolizumab. It needs to be noted that 97.1% of patients had been previously treated with at least one TNFi.
All patients included in this study completed the induction phase at last observation; the mean follow-up of the entire cohort was 14.3 months. Of the patient population, 8.2% (14 patients) had a history of IBD-associated SpA (according to ASAS criteria) at the time of initiation of vedolizumab. 5.8% (10 patients) were in clinical remission regarding SpA, while 2.4% (4 patients) had active SpA when they initiated vedolizumab. First, no clinical benefit on SpA following initiation of vedolizumab was found in those 4 patients with active SpA. Second, exacerbation of SpA in patients with clinical remission at initiation of vedolizumab was found in 6/10 patients whereas no effect was reported in the remaining 4/10 patients. All 14 patients with IBD-associated SpA were under TNFi just before starting vedolizumab. Finally, new onset of SpA induced by vedolizumab was reported in 1 patient (see Table).[1]
Table: Characteristics of patients and the main results [1]
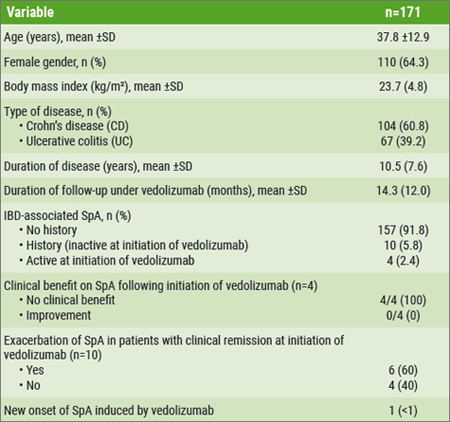
Dr. Paccou (Amiens University Medical Center, France) concluded that this study of vedolizumab does not support its efficacy in patients with IBD-associated SpA. It might even induce exacerbation or new onset of SpA. However, inception cohort studies are needed to prospectively evaluate the effect of vedolizumab on joint manifestations.[1] Overall, this study provides good translational therapeutic data supporting the concept that the pathomechanisms of joint manifestations in SpA and IBD differ, at least in a proportion of subjects.
- Paccou J, et al. Abstract OP0029. EULAR 2018.
Posted on
Previous Article
« Osteoporosis and vertebral fractures associated with disease activity/radiographic damage in axial spondyloarthritis Next Article
Novel systemic sclerosis drugs: an overview of what might be to come »
« Osteoporosis and vertebral fractures associated with disease activity/radiographic damage in axial spondyloarthritis Next Article
Novel systemic sclerosis drugs: an overview of what might be to come »
Table of Contents: EULAR 2018
Featured articles
Rheumatoid Arthritis
Switching to biosimilar bDMARDs is safe and efficacious
No significant differences when tapering TNF blockers versus csDMARDs
Confirmation of long-term safety profile adalimumab across indications
Ankylosing Spondylitis
Clinical effect of vedolizumab on articular manifestations spondyloarthritis associated with IBD
Synergistic effect NSAIDs plus TNFi in slowing radiographic progression in ankylosing spondylitis patients
Osteoporosis and Osteoarthritis
Systemic Sclerosis and Systemic Lupus Erythematosus
Promising results rituximab in systemic sclerosis, and systemic lupus erythematosus classification criteria
Related Articles

June 22, 2022
EULAR 2022 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

