The association of biologic drug levels with infection risk
With the availability of TNF drugs, higher doses of anti-TNF agents have been reported to be associated with an increased serious infection risk.[13] Yet, no registries have systematically evaluated the effect of drug levels on infection risk. Jani et al. assessed the effect of biologic drug levels in RA patients on all infections and serious infections, defined as infections that require hospitalisation, intravenous antibiotics, or which lead to death.[14]
A total of 703 patients recruited for the British Society for Rheumatology Biologics Register-RA (safety data) and the Biologics in RA Genetics & Genomics Syndicate (serological samples) were included. The cohort included 286 patients using etanercept, 179 adalimumab, 120 certolizumab, 104 tocilizumab, and 14 infliximab. The majority (74%) were women, the mean age was 58 years, and 89% was on a first biologic.
Biologic drug levels were measured at 3, 6, and 12 months after biologic initiation and stratified as low/normal or high drug levels, as per thresholds defined using concentration-effect curves for each drug. The crude rate/1000 patient-years was 314 and 464 for all infections, and 54 and 76 for serious infections in the low/normal and high drug level groups, respectively. The adjusted hazard ratio for all infections within the first year differed significantly between the two groups; the high drug level group had a 50% higher risk of all infections (HR: 1.51; 95% CI 1.14, 2.01).14 The most common types of all infections in the high drug level group were lower (34%) and upper (16%) respiratory tract infections, urinary tract infections (15%), and skin infections including shingles (8%).
It was concluded that RA patients with high biologic drug levels have a higher risk of infection and that monitoring drug levels may be helpful in prediction of infection. For patients with high drug levels in disease remission, biologic dose tapering may lower infection risk.[14]
Clinical effectiveness of sarilumab in rheumatoid arthritis patients with TNFi failure
Sarilumab, a human monoclonal antibody, binds membrane and soluble interleukin (IL)-6 and was recently approved for the treatment of moderate-to-severe RA. The TARGET (NCT10709758) trial showed that sarilumab 150 mg and 200 mg q2w plus cDMARDs was clinically efficacious in RA patients with an inadequate response or intolerance to TNF inhibitors (TNFi).15 In a post-hoc analysis of this trial, researchers assessed whether improvements in PROs differ between patients with primary or secondary TNFi failures prior to sarilumab treatment. Efficacy results of sarilumab after 24 weeks of treatment are outlined in the Table.
Table: Least square mean changes at week 24 in PROs with sarilumab and placebo following TNFi failure [15]
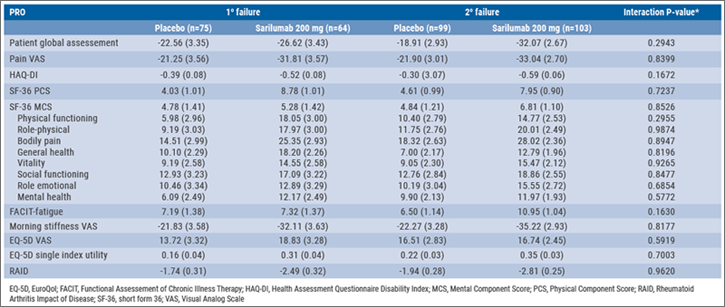
In the TARGET study, 92.3% of patients failed prior TNFi, of which 38.6% had primary TNFi failure, and 53.7% had secondary TNFi failure. At week 24, numerical improvements were reported in all PROs for both the 150 and 200 mg sarilumab groups. Changes in all PROs were numerically similar in the primary and secondary TNFi failure patients with sarilumab 200 mg vs placebo at week 24. Furthermore, treatment-by-subgroup interaction testing did not show a statistically significant interaction of TNFi failure status and PRO outcome (all interaction P>0.05). Treatment-emergent AEs occurred in 59.7% of 150 mg sarilumab, 65.6% of 200 mg sarilumab, and 45.3% of placebo patients in the primary failure group. In the secondary failure group this was 73.6% of 150 mg sarilumab, 63.1% of 200 mg sarilumab, and 52.5% of placebo patients.
Safety data were consistent with IL-6 receptor blockade with tocilizumab and the previously reported safety profile of sarilumab. Thus, sarilumab 150 mg and 200 mg q2w are clinically effective in treating TNFi failure in RA patients. Both led to improvements in PROs and changes in these PROs were similar regardless of whether patients had experienced primary or secondary TNFi failure.[15]
- Singh JA, et al. Lancet. 2015;368:258-265.
- Jani M, et al. Abstract OP0229. EULAR 2018.
- Strand V, et al. Abstract FRI0144. EULAR 2018.
Posted on
Previous Article
« Confirmation of long-term safety profile adalimumab across indications Next Article
Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey »
« Confirmation of long-term safety profile adalimumab across indications Next Article
Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey »
Table of Contents: EULAR 2018
Featured articles
Rheumatoid Arthritis
Switching to biosimilar bDMARDs is safe and efficacious
No significant differences when tapering TNF blockers versus csDMARDs
Confirmation of long-term safety profile adalimumab across indications
Ankylosing Spondylitis
Clinical effect of vedolizumab on articular manifestations spondyloarthritis associated with IBD
Synergistic effect NSAIDs plus TNFi in slowing radiographic progression in ankylosing spondylitis patients
Osteoporosis and Osteoarthritis
Systemic Sclerosis and Systemic Lupus Erythematosus
Promising results rituximab in systemic sclerosis, and systemic lupus erythematosus classification criteria
Related Articles
January 18, 2021
Inflammatory disease as a risk factor for fractures
February 4, 2020
Depression closely related to fatigue in SLE patients
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

