The main results of DAPA-HF trial were presented by Prof. John J. McMurray (Cardiovascular Research Centre, Scotland) [1]. The goal of this phase 3, randomised, double-blinded trial was to evaluate 10 mg/day dapagliflozin (a sodium-glucose cotransporter 2 [SGLT2] inhibitor) + standard therapy versus placebo + standard therapy in HFrEF patients. Specifically, investigators wanted to determine whether adverse outcomes could be prevented in non-diabetic patients in addition to patients with T2DM. The results were recently published in the New England Journal of Medicine [2]. Two additional prespecified sub-analyses, looking at age and health status, were also presented at the AHA Scientific Sessions [3,4].
The primary outcome was the composite of CV death, hospitalisation for heart failure, or urgent heart failure visit. Key secondary outcomes were separate evaluation of CV death, hospitalisation for heart failure, and worsening of renal function.
In total, 4,744 HFrEF patients with and without T2DM were enrolled with a median follow-up of 18.2 months. Primary outcome events occurred in 16.3% of the dapagliflozin group compared with 21.2% of the placebo group (P<0.001), which translated to a reduction of 26% for the combined endpoint of CV death and worsening of heart failure, alongside standard of care (see Table).
Table: Relative benefit of dapagliflozin as compared with placebo in patients with and without type 2 diabetes mellitus
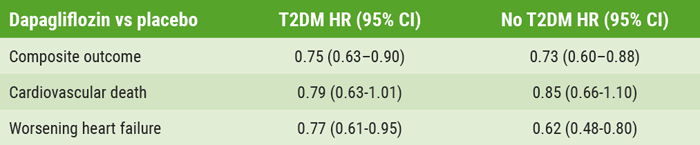
HR, hazard ratio; T2DM, type 2 diabetes mellitus.
The DAPA-HF cohort included 2,139 patients with T2DM (45%) and 2,605 without T2DM. Those with T2DM had a 21% reduction in CV death with dapagliflozin versus placebo, while the reduction was 15% in patients without diabetes. The relative benefit was reversed in worsening of heart failure: among patients with T2DM, there was a 23% reduction in events versus placebo, while there was a 38% reduction in heart failure events among those without diabetes.
Prof. Felipe A. Martinez (National University of Córdoba, Spain) presented a sub-analysis aimed to examine the effects of age on response to dapagliflozin versus placebo in the DAPA-HF cohort, which spanned the ages of 22-94 years [3]. The benefits of dapagliflozin were evident across all age groups when compared with placebo: 636 patients were <55 years old (13.4%; HR 0.87; 95% CI 0.60-1.28); 1,242 were ages 55-64 (26.2%; HR 0.71;95% CI 0.55-0.93); 1,717 were ages 65-74 (36.2 %; HR 0.76; 95% CI 0.61-0.95); and 1,149 were >75 years (24.2%; HR 0.68; 95% CI 0.53-0.88). The interaction between age and response was not significant (Pinteraction=0.76). Results showed that the rate of the primary outcome –a composite of an episode of worsening heart failure or CV death– in each age group in the placebo group was 13.6%, 15.7%, 15.1%, and 18.0%, respectively, versus the dapagliflozin group: 11.8%, 11.4%, 11.4%, and 12.6, respectively [5]. Prof. Martinez said that "dapagliflozin was well tolerated, with no significant difference between dapagliflozin and placebo in any age group,” and that the effects were consistent "both in terms of efficacy and safety." The investigators concluded that dapagliflozin has substantial clinical benefits in older as well as younger patients.
In a separate analysis of the DAPA-HF cohort, presented by Prof. Mikhail N. Kosiborod (Saint Luke's Mid America Heart Institute, USA), dapagliflozin was compared with placebo based on health status as determined by the Kansas City Cardiomyopathy Questionnaire (KCCQ) [4,6]. Data was available for 4,443 patients. Results showed that dapagliflozin versus placebo was associated with a 2.3-point increase in KCCQ overall summary score from baseline to 8 months (P<0.0001). However, dapagliflozin benefits were consistent across all tertiles of KCCQ total symptom scores: lowest tertile (HR 0.70; 95% CI 0.57-0.86), middle tertile (HR 0.77; 95% CI 0.61-0.98), and highest tertile (HR 0.62; 95% CI 0.46-0.83), with no significant association with any of the 3 tertiles individually (Pheterogeneity=0.52).
In conclusion, dapagliflozin was beneficial for symptomatic HFrEF patients and was associated with a reduction in CV deaths and heart failure events and improvement in symptoms. Age, diabetes, and baseline health status did not influence the benefit of dapagliflozin. There were no safety signals of concern. These trials will likely change the practice of treating patients with HFrEF with dapagliflozin.
- McMurray JJ, et al. The dapagliflozin and prevention of adverse-outcomes in heart failure trial (DAPA-HF): results in non-diabetic patients. LBS.05, AHA Scientific Sessions 2019, 14-18 November, Philadelphia, USA.
- McMurray JJ, et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Nov 21;381(21):1995-2008.
- Martinez FA, et al. DAPA-HF- Effect of treatment based on age in the dapagliflozin and prevention of adverse-outcomes in heart failure. LBS.05, AHA Scientific Sessions 2019, 14-18 November, Philadelphia, USA.
- Kosiborod MN, et al. DAPA-HF- Effect of treatment measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) in the dapagliflozin and prevention of adverse-outcomes in heart failure. LBS.05, AHA Scientific Sessions 2019, 14-18 November, Philadelphia, USA.
- Martinez FA, et al. Efficacy and Safety of Dapagliflozin in Heart Failure With Reduced Ejection Fraction According to Age. Circulation. 2020;141:100–111.
- Kosiborod MN, et al. Effects of Dapagliflozin on Symptoms, Function, and Quality of Life in Patients With Heart Failure and Reduced Ejection Fraction: Results From the DAPA-HF Trial. Circulation. 2020 Jan 14;141(2):90-99.
Posted on
Previous Article
« ORION-9: Inclisiran RNAi halves LDL in familial hypercholesterolaemia patients Next Article
Balloon-expandable better than self-expanding transcatheter heart valves »
« ORION-9: Inclisiran RNAi halves LDL in familial hypercholesterolaemia patients Next Article
Balloon-expandable better than self-expanding transcatheter heart valves »
Table of Contents: AHA 2019
Featured articles
New Approaches to CVD Risk Reduction
Phase 3 BETonMACE trial did not meet its primary endpoint
Inclisiran safely halves LDL-Cholesterol
Colchicine prevents cardiovascular events
Interventional Management for Acute Coronary Syndrome
Drop aspirin after 3 months in non-STEMI ACS patients on dual antiplatelet therapy
Immediate coronary angiography after cardiac arrest does not improve survival
Complete revascularisation for obstructive non-culprit lesions with vulnerable plaque
Colchicine: no difference in peri-procedural cardiovascular events 30 days post-PCI
Intra-aortic balloon pump better than Impella: new observational data
Results for the Ischemia Trials: To Intervene or Not to Intervene
ISCHEMIA trial: Invasive treatment only better for angina burden
Controversies in Contemporary Management of Aortic Stenosis
Full GALILEO results: Why did rivaroxaban fail after TAVR?
Balloon-expandable better than self-expanding transcatheter heart valves
RECOVERY: Benefit of early surgery in asymptomatic severe aortic stenosis
Guidelines: Updates and Controversies
New guidelines on the prevention of cardiovascular conditions
Trials in Electrophysiology and Left Ventricular Function
RENAL-AF trial: Apixaban similar to warfarin
Apple Heart Study: Not just for atrial fibrillation
Early apixaban safe as secondary prevention of stroke from AF
Carvedilol does not improve exercise performance in Fontan patients
New Frontiers in Lipid Therapy
Icosapent ethyl plus statins reduces total plaque volume
ORION-9: Inclisiran RNAi halves LDL in familial hypercholesterolaemia patients
New RNAi therapies to reduce triglycerides: 2 studies show favourable results
Targeting LDL-C <70 mg/dL is better than 100 mg/dL after stroke
Challenges in Heart Failure Management
FUEL trial: Udenafil improves some exercise measurements in Fontan
DAPA-HF: Dapagliflozin also good for heart failure patients without diabetes, of any age, or any health status
PARAGON-HF: Benefits for women and lower ejection fraction
Related Articles
February 26, 2020
Balloon-expandable better than self-expanding transcatheter heart valves
February 26, 2020
Full GALILEO results: Why did rivaroxaban fail after TAVR?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

