The working hypothesis was that P2Y12 inhibitor monotherapy after 3 months of DAPT would be non-inferior to 12 months of DAPT at 12 months after the index procedure [1].
This prospective, multicentre, randomised, open-label, non-inferiority trial included almost 3,000 patients (mean age 65 years, 27% female) who underwent percutaneous coronary intervention (PCI) with DES. They were randomised 1:1 to either DAPT for 3 months (n=1,495) or 12 months (n=1,498). In the former group, after 3 months, aspirin was discontinued and P2Y12 inhibitor monotherapy was continued. Stratification was performed by type of DES used (i.e. cobalt-chromium everolimus-eluting stent vs platinum-chromium everolimus-eluting stent vs biodegradable polymer sirolimus-eluting stent). The primary endpoint was 12-month major adverse cardiac and cerebrovascular events (MACCE; a composite of all-cause death, myocardial infarction, or stroke). The mean duration of aspirin was 96 days in the P2Y12 inhibitor group and 365 days in the DAPT group.
The results showed that the primary outcome (MACCE) at 12 months was 2.9% for 3 months of DAPT vs 2.5% for 12 months of DAPT (P=0.007 for non-inferiority; P=0.46 for superiority). The rate for all-cause death was 1.4% vs 1.2% (P=0.61), respectively, and for MI 0.8% vs 1.2% (P=0.28) (see Table).
Table: Clinical outcomes after 3 months vs 12 months of dual antiplatelet therapy [1]
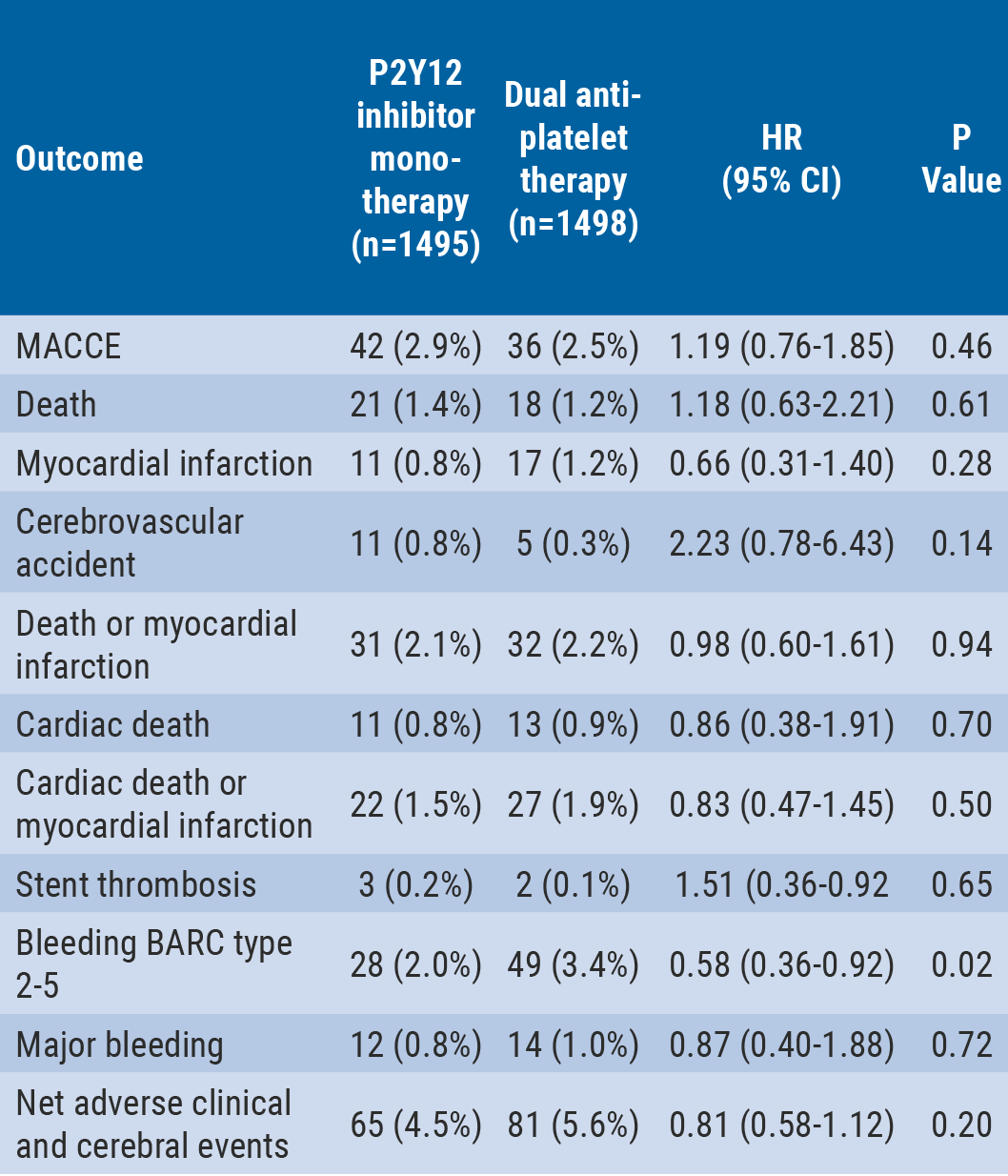
Major bleeding was defined as BARC type 3-5 bleeding. Net adverse clinical and cerebral events were defined as MACCE plus BARC type 2-5 bleeding.
The authors concluded that in this prospective, open-label, randomised trial, P2Y12 inhibitor monotherapy after 3 months of DAPT was non-inferior to 12 months of DAPT for the primary endpoint of MACCE at 12 months after the index procedure. The 3-month landmark analysis and per-protocol analysis showed consistent results. Moreover, P2Y12 inhibitor monotherapy was associated with lower risk of bleeding compared with prolonged DAPT. Although these results need to be validated in well powered blinded trials, these data raise the hypothesis that P2Y12 inhibitor monotherapy after short duration of DAPT may be a novel antiplatelet strategy balancing ischaemic and bleeding risk in patients undergoing PCI.
1. Hahn J-Y, et al. Abstract 410-16. ACC 2019, 16-18 March, New Orleans, USA.
Posted on
Previous Article
« Treatment patterns PAH have changed recently Next Article
BNP still a reliable prognostic marker before and during sacubitril/valsartan treatment »
« Treatment patterns PAH have changed recently Next Article
BNP still a reliable prognostic marker before and during sacubitril/valsartan treatment »
Table of Contents: ACC 2019
Featured articles
Acute and Stable Ischaemic Heart Disease
Arrhythmias and Clinical Electrophysiology
Substantial impact of temporary interruptions of warfarin versus DOAC
Smartwatch can detect atrial fibrillation with high degree of accuracy
Congenital Heart Disease
Heart Failure and Cardiomyopathies
Frequent use of beta-blocker after HFpEF hospitalisations in elderly patients without compelling indications
High 5-year survival rates for older HF patients without initial severe comorbidity
Pulmonary Arterial Hypertension and Venous Thromboembolism
Interventional Cardiology
Vascular Medicine
Lower rates stroke/SE with DOACs in frail non-valvular AF patients
Similar rates of stroke/SE associated with DOAC vs warfarin use in obese non-valvular AF patients: Results from an observational registry
Convincing evidence of the role of icosapent in reducing subsequent CV events
Related Articles

June 6, 2019
Treatment patterns PAH have changed recently
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

