https://doi.org/10.55788/5f9c17ec
“Hidradenitis is a horrible disease and we're constantly searching for new and improved therapies,” Dr Kim Papp (Probity Medical Research, ON, Canada) acknowledged [1]. He presented the 12-week results of an ongoing phase 3 trial (NCT05905783) on izokibep, a small protein that inhibits IL-17A.
The study assessed efficacy and safety in moderate-to-severe HS and randomised 258 participants (mean age of 37.3 years; 69% women) to weekly subcutaneous injections with 160 mg of izokibep or placebo over 16 weeks. The mean count of abscesses and nodules was 13.4, draining tunnels count 2.2, the Dermatology Life Quality Index (DLQI) was 11.9, and the disease duration was over 10 years.
A first statistically significant separation of the primary endpoint, an improvement of the HS clinical response by 75% (HiSCR75) curves was already seen at week 4. At week 12, 33% of participants in the izokibep arm reached HiSCR75 versus 21% on placebo (P<0.05) (see Figure). Only moments before his presentation, Dr Papp received the official results of HiSCR75 at week 16. “You will see it in the final publication, week 16: 38% izokibep and 20% placebo—spectacular results.”
Figure: Percentage of participants reaching the primary endpoint of HiSCR75 at week 12 [1]
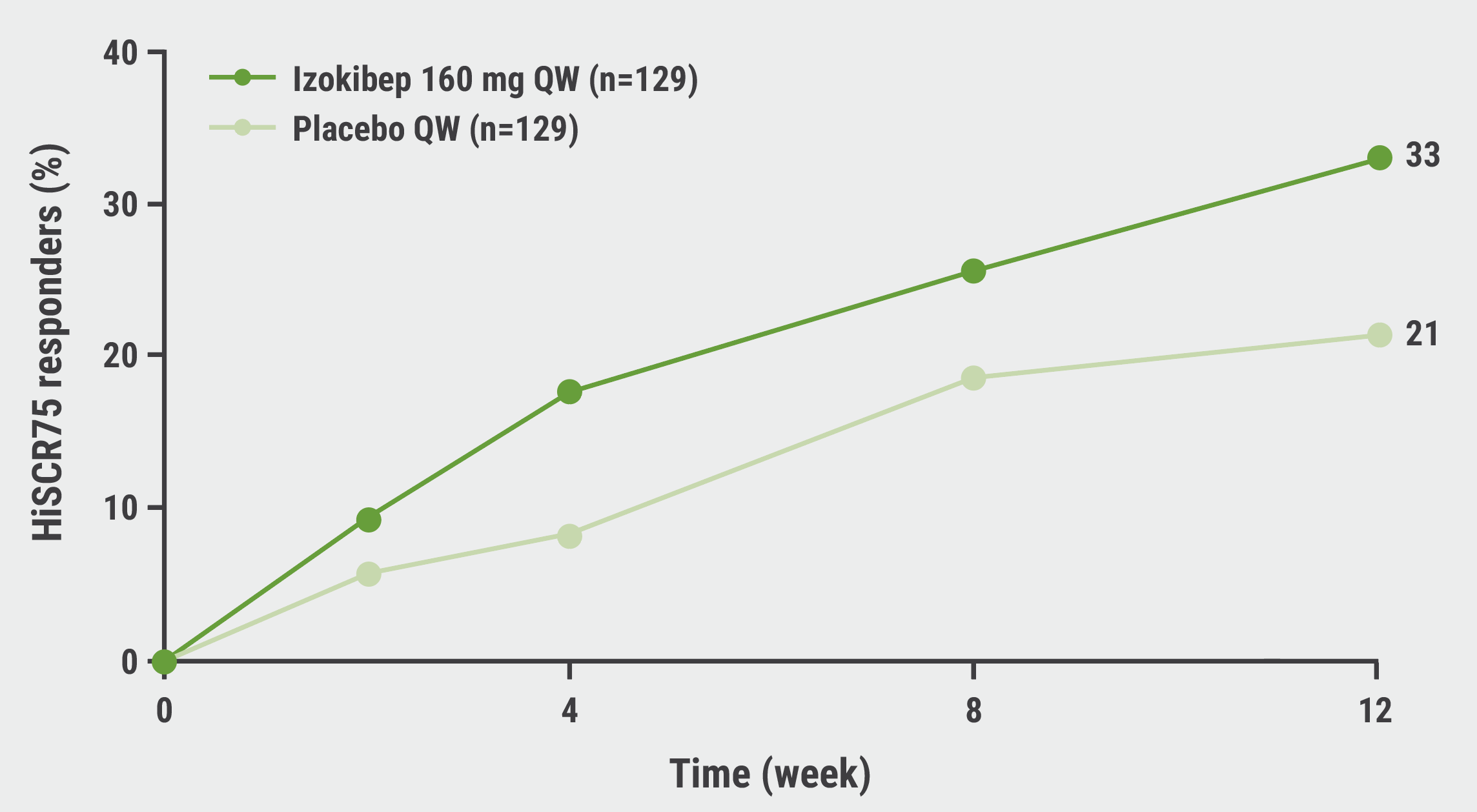 HiSCR75, Hidradenitis suppurativa clinical response improvement by 75%; QW, once weekly.
HiSCR75, Hidradenitis suppurativa clinical response improvement by 75%; QW, once weekly. The secondary endpoints HiSCR90 and HiSCR100 also favoured izokibep (25% vs 9%; P<0.001 and 22% vs 8%; P<0.01). Further ameliorations were found in patient-reported outcomes like DLQI and skin pain numeric rating scale (Skin Pain-NRS). Of note, half of the baseline Hurley stage II participants attained a count of 0, 1, or 2 for inflammatory lesions (P<0.01).
The interim rate of serious treatment-emergent adverse events was 0.8% on izokibep and 3.1% on placebo. The most common adverse events were injection-site reaction (65.1% vs 7.8%), headache (10.1% vs 9.3%), and nasopharyngitis (7.0% in both groups). No cases of candidiasis were seen in the izokibep arm.
- Papp KA, et al. Efficacy and safety of izokibep, a novel IL-17A inhibitor, in moderate-to-severe hidradenitis suppurativa: Week 12 results from a randomized, double-blind, placebo-controlled, multicentre, phase 3 study. D1T01.2A, EADV Congress 2024, 25–28 September, Amsterdam, the Netherlands.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Bimekizumab shows sustained 2-year efficacy in hidradenitis suppurativa Next Article
Atopic hand eczema: similar treatment success for dupilumab and topical delgocitinib »
« Bimekizumab shows sustained 2-year efficacy in hidradenitis suppurativa Next Article
Atopic hand eczema: similar treatment success for dupilumab and topical delgocitinib »
Table of Contents: EADV 2024
Featured articles
Delgocitinib cream outperforms oral alitretinoin in chronic hand eczema
News in Atopic Dermatitis
3-Year results highlight durable effects of IL-13 inhibitor in AD
IL-22RA1 inhibition shows potential in atopic dermatitis
Lifelong psychosocial burden linked to early-onset atopic dermatitis
Second-generation selective PDE4 inhibitor shows promise in AD
What’s New in Prurigo Nodularis and Lichen Planopilaris
Prurigo nodularis: long-term treatment decreases relapse events
JAK1 inhibitor shows promising long-term efficacy in PN
Hand Eczema: End of the Therapeutic Draught
Delgocitinib cream outperforms oral alitretinoin in chronic hand eczema
Atopic hand eczema: similar treatment success for dupilumab and topical delgocitinib
Hidradenitis Suppurativa: New Medications on the Horizon
Targeting IL-17A offers a promising treatment perspective in hidradenitis suppurativa
Bimekizumab shows sustained 2-year efficacy in hidradenitis suppurativa
Familial hidradenitis suppurativa tied to metabolic disease
Psoriasis in 2024
Imsidolimab potential future therapeutic avenue for generalised pustular psoriasis
A new era of care: Artificial intelligence in psoriasis
New Developments in Hair Disorders
Deuruxolitinib significantly improves hair satisfaction in AA
Topical pan-JAK inhibitor mitigates inflammatory biomarkers in frontal fibrosing alopecia
Miscellaneous
Vitiligo: Prolonged facial re-pigmentation maintained with continued ruxolitinib cream
Anti-KIT antibody: the next frontier in CSU treatment?
New targets identified for acute and chronic wound healing
Interesting Posters
PsoBest registry: Biologics dominate treatment for moderate-to-severe psoriasis
Semaglutide improves outcomes for patients with obesity and HS
Advanced BCC: histological subtype and time to complete response may predict tumour recurrence
Related Articles
August 13, 2021
First-in-class gel shows promise for basal cell carcinoma
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

