“Gemcitabine/cisplatin chemotherapy has been the first-line standard-of-care for patients with advanced biliary tract cancer (BTC) for over a decade,” Dr Do-Youn Oh (Seoul National University College of Medicine, South Korea) explained. “However, the prognosis for these patients is poor. Durvalumab is a PD-L1 inhibitor that has demonstrated encouraging anti-tumour activity in patients with advanced BTC in a phase 2 study” [2]. The double-blind, placebo-controlled, phase 3 TOPAZ-1 trial (NCT03875235) randomised 685 patients 1:1 to 8 cycles of gemcitabine/cisplatin chemotherapy plus durvalumab 1,500 mg every 3 weeks followed by durvalumab 1,500 mg every 4 weeks, or gemcitabine/cisplatin plus placebo. Overall survival (OS) was the primary endpoint.
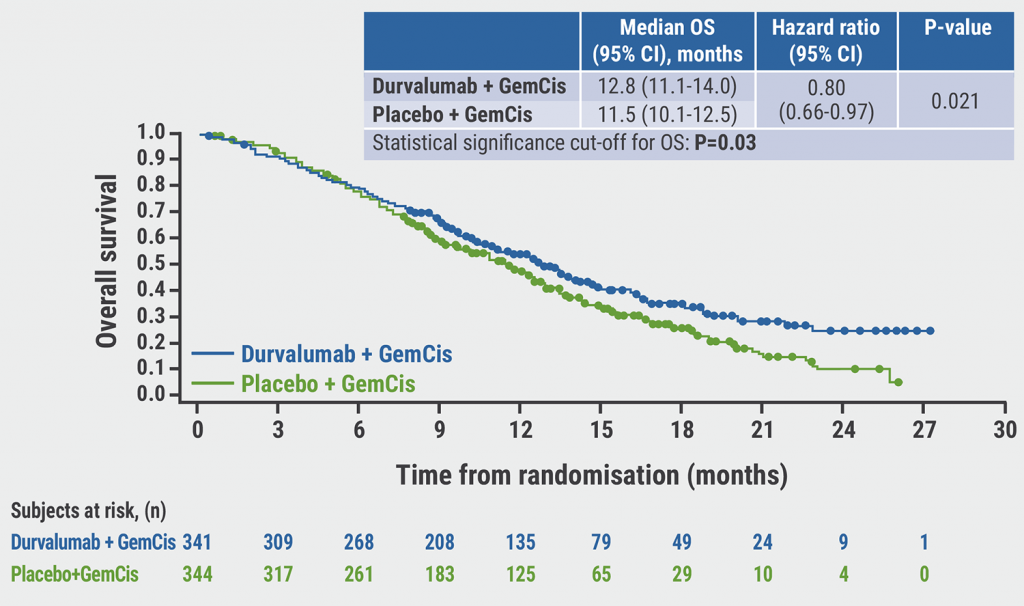
Patients receiving durvalumab had a higher median OS than patients receiving placebo (12.8 vs 11.5 months; HR 0.80; P=0.021; see Figure). Notably, the absolute OS differences showed that the effect increased with time from randomisation: 12-month OS was 6.1%, 18-month OS 9.5%, and 24-month OS 14.5%. OS subgroup analysis displayed consistency across pre-defined strata. Furthermore, the progression-free survival data favoured the durvalumab arm over the placebo arm (median 7.2 vs 5.7 months; P=0.0001).
Figure: Overall survival analysis of TOPAZ-1 [1]
The safety analysis revealed similar toxicity profiles for the 2 treatment arms. The observed adverse events (AEs) were in line with the known safety profile of gemcitabine/cisplatin chemotherapy. Immune-related AEs occurred more frequently in patients on durvalumab than in patients on placebo (12.7% vs 4.7%). Hypothyroid events and dermatitis/rash were the most commonly observed immune-related AEs. Fortunately, grade 3 or higher immune-related AEs were rare (durvalumab 2.4% vs placebo 1.5%).
- Oh D, et al. A phase 3 randomized, double-bind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin in patients with advanced biliary tract cancer: TOPAZ-1. Abstract 378, ASCO GI 2022, 20–22 January.
- Oh D, et al. Abstract 4520, ASCO Annual Meeting 2020, 29–31 May.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« HIMALAYA: Durvalumab ± tremelimumab new first-line option for unresectable HCC Next Article
EPOCH trial: Subgroup analyses and additional endpoints for TARE plus chemo »
« HIMALAYA: Durvalumab ± tremelimumab new first-line option for unresectable HCC Next Article
EPOCH trial: Subgroup analyses and additional endpoints for TARE plus chemo »
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

