Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapies approved in the USA and EU for adults with relapsed/refractory (R/R) LBCL after ≥2 lines of systemic therapy. “The approval was based on the JULIET and ZUMA-1 trials,” Dr Emmanuel Bachy (Hospices Civils de Lyon, France) mentioned.
Two pivotal trials, but no direct comparisons
In the JULIET trial, tisa-cel was associated with a best overall response rate (ORR) of 52% with a 40% complete response rate (CRR) and a median overall survival (OS) of 12 months [2]. In the ZUMA-1 trial, axi-cel an led to a 83% ORR, a 58% CRR and a median OS of 26 months [3]. Real-world data have confirmed efficacy and manageable toxicity for both products [4,5].
The absence of an individual patients data-based comparison from both trials has precluded a direct comparison of outcome and toxicity for axi-cel and tisa-cel. Matched-adjusted indirect comparisons have produced conflicting results [6]. DESCAR-T is the French registry for CAR T-cell therapy, required by the Health Authority.
Large cohort from France
Dr Bachy and others conducted a propensity score-matched comparison of axi-cel and tisa-cel in a large cohort of R/R DLBCL patients treated outside of clinical trials. The initial cohort of 504 patients with DLBCL was treated with axi-cel (n=321) or tisa-cel (n=183). Among others, patient characteristics were imbalanced regarding ECOG, and prior transplant rate with a worse prognosis for patients receiving tisa-cel.
After a 1:1 ratio propensity score-matching, the outcome was compared between 144 patients treated with axi-cel and 144 patients treated with tisa-cel. There was no residual significant difference in baseline patient characteristics according to CAR T-cell type.
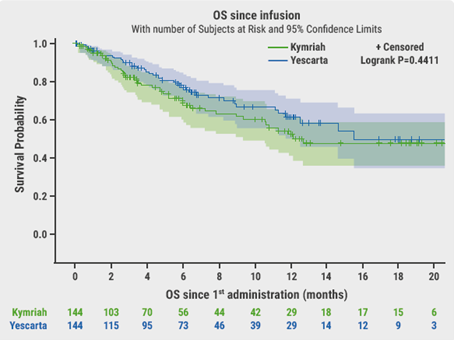
The primary endpoint was OS. After a median follow-up of 6.6 months, OS was not significantly different between axi-cel and tisa-cel at 6 months (78% vs 70% respectively, P=0.44, Figure). Best ORR and CRR were significantly higher with axi-cel compared with tisa-cel (73% vs 60%, P=0.02 and 56% vs 36%, P<0.001, respectively). There was no difference in duration of response (DoR). At 6 months, PFS was significantly longer with axi-cel (53%) than with tisa-cel (32%, P=0.011).
With respect to the toxicity profile, there was no significant difference in the incidence of cytokine release syndrome (CRS), but axi-cel was associated with significantly more frequent neurotoxicity (ICANS) compared with tisa-cel, namely:
- 6% vs 18.1% for grade 1-2 ICANS; and
- 4% vs 2.1% for grade ≥3 ICANS (P<0.001).
Conclusion
After stringent propensity score-matching on a large patient population treated with CAR T-cell therapy, axi-cel resulted in higher response rates and significantly prolonged PFS compared with tisa-cel. However, greater efficacy came at the cost of somewhat higher neurotoxicity with axi-cel. There was no difference in DOR and OS, but longer follow-up is needed between axi-cel and tisa-cel.
These data could help in refining CAR T-cell subtype choice for different patient populations, with young and/or fit patients benefiting most from axi-cel, while tisa-cel being most advantageous to elderly and/or unfit patients.
- Bachy E, et al. A Propensity Score-Matched Comparison of Axi-Cel and Tisa-Cel for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Real-Life: A Lysa Study from the Descar-T Registry. Abstract 92, ASH 2021 Annual Meeting 11-14 Dec.
- Schuster SJ, et al. N Engl J Med. 2019;380:45-56.
- Neelapu SS, et al. N Engl J Med. 2017;377:2531-2544.
- Jacobson CA, et al. J Clin Oncol. 2020;38:3095-3106.
- Nastoupil LJ, et al. J Clin Oncol. 2020;38:3119-3128.
- Oluwole OO, et al. Biol Blood Marrow Transplant. 2020;26:1581-1588.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma Next Article
CIRS is predictive of outcomes in CAR T-cell recipients with R/R DLBCL »
« Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma Next Article
CIRS is predictive of outcomes in CAR T-cell recipients with R/R DLBCL »
Table of Contents: ASH 2021 Focus on CAR T-Cell Therapy
Featured articles
Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma
CAR T-cell Therapy
Most re-hospitalisations within first month from CAR T-cell infusion
CD22-directed CAR T-cell therapy safe and well-tolerated in R/R LBCL
High rate of rapid and complete responses with axi-cel in high-risk large B-cell lymphoma
Novel anti-CD19 plus lenalidomide prolonged survival in R/R DLBCL
Liso-cel superior to standard-of-care as second-line therapy in large B-cell lymphoma
CIRS is predictive of outcomes in CAR T-cell recipients with R/R DLBCL
Axi-cel more effective but tisa-cel less toxic in large B-cell lymphoma
Axi-cel improved event-free survival in relapsed/refractory large B-cell lymphoma
Comparable outcomes with second-line tisa-cel versus standard-of-care for relapsed/refractory aggressive NHL
Improved QoL with axi-cel versus standard-of-care in R/R LBCL
Related Articles

September 14, 2023
How can we further improve lymphoma care?

June 26, 2023
ASCO 2023 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

