https://doi.org/10.55788/d5098015
COLCHICINE-PCI studied pre-procedural use of colchicine in patients with ischaemic heart disease or acute coronary syndrome undergoing PCI. The primary endpoint —myocardial injury, as determined by troponin I concentration— was not different between the 2 arms. In total, 118 subjects assigned to the colchicine arm (57.3%) versus 122 patients in the placebo arm (64.2%) experienced a primary outcome event (P=0.19). Furthermore, no statistically significant distinctions were observed between the colchicine arm versus the placebo arm for any of the secondary endpoints, i.e. peri-procedural myocardial injury using CKMB and the SCAI definition of clinically relevant myocardial infarction after coronary revascularisation or the occurrence of major adverse cardiac events (MACE) with composite of the earliest occurrence of death from any cause, non-fatal myocardial infarction (defined by the Universal Definition), or target vessel revascularisation (bypass surgery or repeat PCI of the target vessel) at 30 days. No differences were observed in all-cause mortality (P=0.49).
In a pre-specified inflammatory marker sub-study with 280 subjects, there was no significant difference for the primary biomarker outcome of soluble interleukin (IL)-6 levels from baseline to 1 hour post-PCI, when comparing the colchicine (n=141) with the placebo (n=139) group. However, at 24 hours post-PCI, IL-6 (76% vs 338%; P=0.02) and high-sensitivity C-reactive protein (7% vs 57%; P=0.001) were both significantly reduced in the colchicine group compared with the placebo group.
In summary, while colchicine appears to attenuate inflammatory cytokine increases during the first 24 hours after PCI, there was no significant difference in peri-procedural myocardial injury or major adverse cardiovascular events at 30 days post-PCI.
1. Shah B, et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: The COLCHICINE-PCI Randomized Trial. LBS04, AHA Scientific Sessions 2019, 14-18 November, Philadelphia, USA.
Posted on
Previous Article
« Phase 3 BETonMACE trial did not meet its primary endpoint Next Article
Carvedilol does not improve exercise performance in Fontan patients »
« Phase 3 BETonMACE trial did not meet its primary endpoint Next Article
Carvedilol does not improve exercise performance in Fontan patients »
Table of Contents: AHA 2019
Featured articles
New Approaches to CVD Risk Reduction
Phase 3 BETonMACE trial did not meet its primary endpoint
Inclisiran safely halves LDL-Cholesterol
Colchicine prevents cardiovascular events
Interventional Management for Acute Coronary Syndrome
Drop aspirin after 3 months in non-STEMI ACS patients on dual antiplatelet therapy
Immediate coronary angiography after cardiac arrest does not improve survival
Complete revascularisation for obstructive non-culprit lesions with vulnerable plaque
Colchicine: no difference in peri-procedural cardiovascular events 30 days post-PCI
Intra-aortic balloon pump better than Impella: new observational data
Results for the Ischemia Trials: To Intervene or Not to Intervene
ISCHEMIA trial: Invasive treatment only better for angina burden
Controversies in Contemporary Management of Aortic Stenosis
Full GALILEO results: Why did rivaroxaban fail after TAVR?
Balloon-expandable better than self-expanding transcatheter heart valves
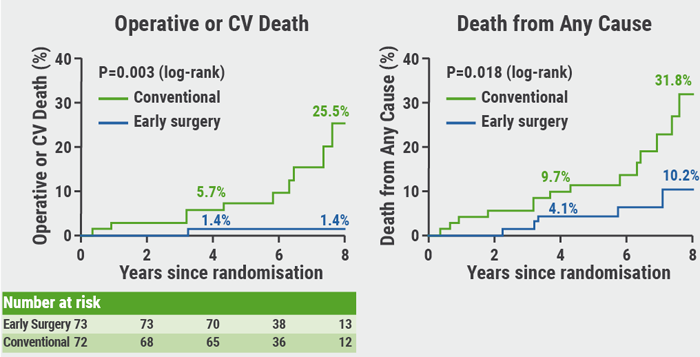
RECOVERY: Benefit of early surgery in asymptomatic severe aortic stenosis
Guidelines: Updates and Controversies
New guidelines on the prevention of cardiovascular conditions
Trials in Electrophysiology and Left Ventricular Function
RENAL-AF trial: Apixaban similar to warfarin
Apple Heart Study: Not just for atrial fibrillation
Early apixaban safe as secondary prevention of stroke from AF
Carvedilol does not improve exercise performance in Fontan patients
New Frontiers in Lipid Therapy
Icosapent ethyl plus statins reduces total plaque volume
ORION-9: Inclisiran RNAi halves LDL in familial hypercholesterolaemia patients
New RNAi therapies to reduce triglycerides: 2 studies show favourable results
Targeting LDL-C <70 mg/dL is better than 100 mg/dL after stroke
Challenges in Heart Failure Management
FUEL trial: Udenafil improves some exercise measurements in Fontan
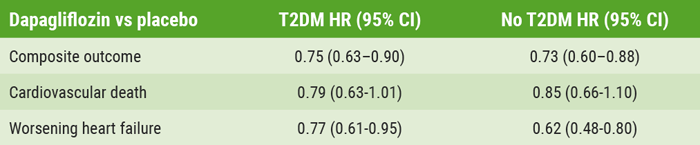
DAPA-HF: Dapagliflozin also good for heart failure patients without diabetes, of any age, or any health status
PARAGON-HF: Benefits for women and lower ejection fraction
Related Articles

February 26, 2020
New guidelines on the prevention of cardiovascular conditions
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

