Stage 1 testicular seminoma has an almost 100% survival rate. Current international guidelines for the management of this cancer recommend CT surveillance of the abdomen/retroperitoneum following orchiectomy, with no adjuvant chemotherapy. While avoidance of chemotherapy avoids the risks and side effects associated with it, there are still long-term adverse health effects resulting from repeated exposure to radiation via multiple CT scans.
Prof. Robert Huddart (Royal Marsden Hospital and Institute of Cancer Research, UK) presented the results of the TRISST trial (NCT00589537) [1]. This phase 3, multicentre, factorial, non-inferiority trial aimed to assess whether CT could be safely replaced with MRI and whether the frequency of imaging could be safely decreased in surveillance of men with stage 1 seminoma. Investigators randomised 669 men to 1 of 4 arms, 2 of which were monitored with CT, and 2 of which were monitored with MRI. Each imaging modality arm had 2 different frequency schedules: one scanned patients at 6, 12, 18, 24, 36, 48, and 60 months; the other scanned patients at 6, 18, and 36 months. Patients randomised to the 3-scan arms only remained there in the absence of disease progression. All patients were followed for 6 years.
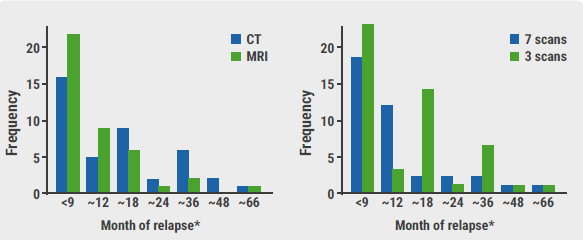
The primary outcome measure was the proportion of patients relapsing with Royal Marsden Hospital stage ≥2C disease. Of the 669 trial participants, 358 (54%) were deemed to be low-risk and 82 (12%) relapsed (see Figure). Only 10 of these 82 were graded as a stage ≥2C relapse. Most relapses were diagnosed at the time of scheduled imaging; additionally, relapse beyond 3 years was rare.
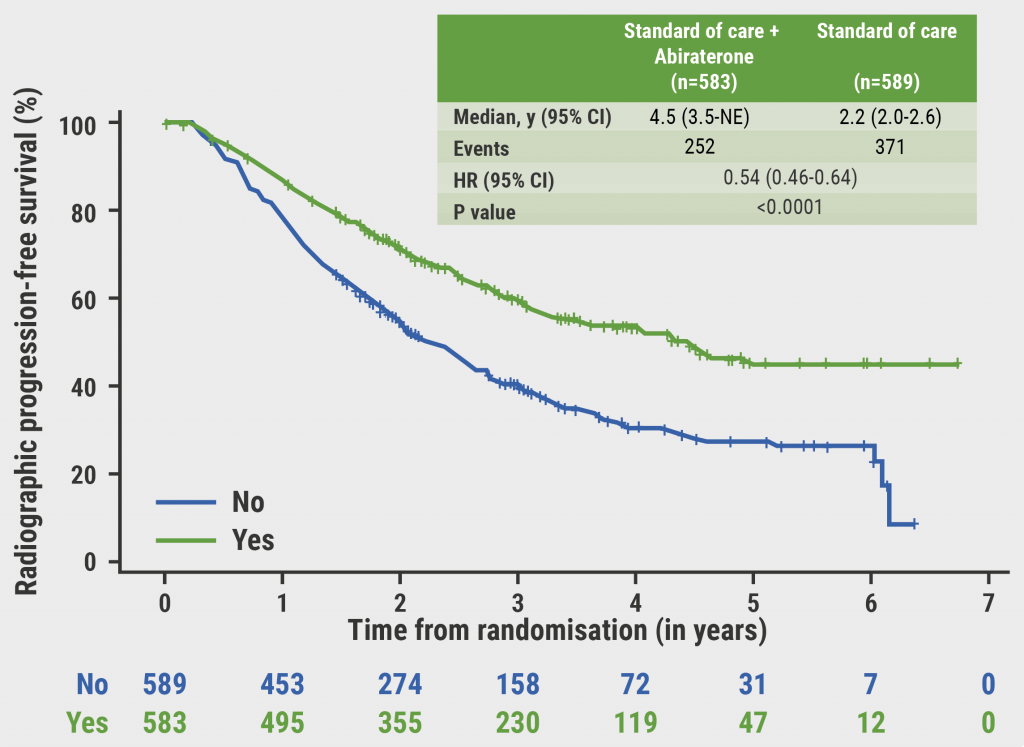
Figure: Relapse frequency for MRI versus CT and 3-scan versus 7-scan schedule [1]
Key secondary outcome measures included disease-free survival and overall survival rates; these were 87% and 99%, respectively. Values were similar across all groups. No tumour-related deaths occurred.
Although more events occurred in those who received 3 scans instead of 7, the criteria for non-inferiority were still met for both the intention-to-treat (ITT) and the per-protocol patients (upper 90% CI <5.7%). There were fewer events in those who received MRI scans than in those who received CT scans; 2 (0.6%) versus 8 (2.5%) (1.9% decrease; 90% CI -3.5% to -0.3% [ITT]). Per protocol results were similar.
Prof. Huddart asserted that surveillance is both safe and effective in stage 1 testicular seminoma, regardless of frequency or type of imaging. Furthermore, imaging beyond 3 years may be unnecessary, as relapse after 3 years is rare. Finally, they recommend that the standard of care should recommend MRI instead of CT, in an attempt to limit radiation exposure in this young population.
- Joffe J. Imaging modality and frequency in surveillance of stage I seminoma testicular cancer: Results from a randomized, phase III, factorial trial (TRISST). Abstract 374, ASCO Genitourinary Cancers Symposium, 11–13 February 2021.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« New therapeutic option for early metastatic seminoma Next Article
New prediction model for brain metastasis in germ cell tumours »
« New therapeutic option for early metastatic seminoma Next Article
New prediction model for brain metastasis in germ cell tumours »
Table of Contents: ASCO GU 2021
Featured articles
Prostate Cancer
Lu177 as a promising new therapy for metastatic prostate cancer
Role of prostate cancer genomics is evolving
Apalutamide prolongs progression-free survival in prostate cancer
Dose-intensified radiation therapy fails to provide better outcomes in prostate cancer
Intrinsic tumour biology may be predictive of treatment response in prostate cancer
Final TITAN trial results favour use of apalutamide
Penile Cancer
Prognosis of penile cancer associated with HPV status
Renal Cancer
Superior clinical outcomes and QoL with nivolumab plus cabozantinib in RCC
Lenvatinib plus pembrolizumab prolongs survival in renal cell carcinoma
Inflammatory markers may guide treatment decisions in metastatic renal cell cancer
Clinical trial exclusion criteria may lead to lack of evidence in real-world patients: how do the excluded fare?
Axitinib offers hope for improving renal cell cancer surgical outcomes
Cabozantinib as possible new first-line therapy in translocation renal cell carcinoma
Predictors of oral anti-cancer agent utilisation in renal cell carcinoma
Denosumab plus pembrolizumab in advanced clear cell renal cell carcinoma
Testicular Cancer
New prediction model for brain metastasis in germ cell tumours
Reduction in radiation exposure is possible in testicular seminoma surveillance
New therapeutic option for early metastatic seminoma
Urothelial Cancer
Poorer outcomes in bladder cancer predicted by race/ethnicity and gender
Enfortumab vedotin as a promising treatment option for bladder cancer: phase 3 results
Enfortumab vedotin as a promising treatment option for bladder cancer: phase 2 results
New standard of care recommended for patients with upper tract urothelial cancer
Signature DNA alterations in subtypes of bladder cancer
ACE inhibitors associated with superior responses in bladder cancer
Better allocation of research dollars needed
Better prediction of favourable responses to immune checkpoint inhibitors in mUC
Genitourinary Oncology
Researchers call for an overhaul of licensing and funding of anti-cancer drugs
Exploring a new strategy for metastatic germ cell tumours
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com