The cell surfaces of prostate cancers strongly express PSMA - even more so in cases of mCRPC. For this reason, PSMA is used both for imaging prostate cancer and as a target for radionuclide therapy.
Dr Michael Hofman (Peter MacCallum Cancer Centre, Australia) discussed the open-label, randomised, multicentre, phase 2 TheraP trial (NCT03392428), which assessed the activity and safety of Lu177-labelled PSMA (LuPSMA) for the treatment of mCRPC [1]. Included men with docetaxel-treated mCRPC and high PSMA expression (n=200) were randomised to receive either ≤6 cycles of radionuclide therapy with LuPSMA or ≤10 cycles of chemotherapy with cabazitaxel.
The primary outcome measure was PSA response rate, defined as the proportion of participants in each group with a ≥50% reduction in PSA from baseline. In the Lu177 group, 65 men (66%) achieved this outcome, compared with 37 men (37%) in the cabazitaxel group (see Figure).
Figure: Primary outcome measure of progression-free survival [1]
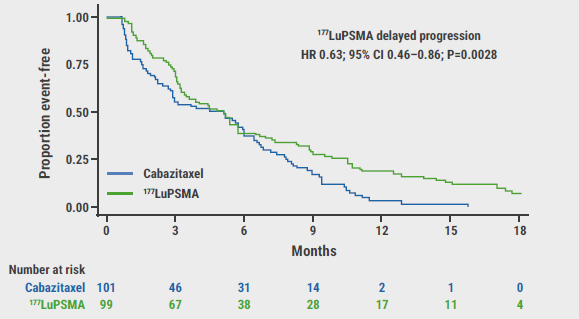
CI, confidence interval; HR, hazard ratio; LuPSMA, lutetium-177-labelled prostate-specific membrane antigen.
Secondary outcome measures included 9 parameters and were analysed after a median follow-up period of 18.4 months. PFS was 19% (95% CI 12–27%) in the radiotherapy group versus 3% (95% CI 1–9%) in the cabazitaxel group (HR 0.63; 95% CI 0.46–0.86; P=0.003; 173 events). Comparable results were found for both radiographic PFS (HR 0.64; 95% CI 0.46–0.88; P=0.007; 160 events) and PSA-PFS, which was defined as the time from randomisation to PSA progression (HR 0.60; 95% CI 0.44–0.83; P=0.002; 172 events). ORR was 49% (95% CI 33–65%) in the Lu177 group and 24% (95% CI 11–38%) in the chemotherapy group (P=0.019). Overall survival will be monitored for 4 years; this data is not yet available since the study only reached its completion date January 2021. To date, 90 deaths are reported. Regarding pain outcomes, 60% of participants in the radiotherapy arm and 43% of participants in the cabazitaxel arm reported pain (RR 1.42; 95% CI 0.84–4.48; P=0.10) at the end of follow-up.
Adverse events (AEs) were monitored from the time of first study dose to 12 weeks after treatment completion. Fewer grade 3 and 4 AEs occurred in the Lu177 group than in the cabazitaxel group (33% vs 53%). The top 3 grade 3 or 4 AEs experienced in the Lu177 group were thrombocytopenia, anaemia, and fatigue; in the cabazitaxel group, they were neutropenia, anaemia, and diarrhoea.
Concerning health-related quality of life outcomes, global health status scores were similar between the groups: LuPSMA was 64 (95% CI 61–67) versus 60 for cabazitaxel (95% CI 57–64). Nonetheless, the Lu177 group reported favourable outcomes compared with the cabazitaxel group in the domains fatigue (34 vs 40; P<0.05), social functioning (79 vs 73; P<0.05), insomnia (24 vs 29; P<0.05), and diarrhoea (8.3 vs 15.6; P<0.001). No domains were favourable in the chemotherapy group.
Dr Hofman concluded that these interim results support the use of LuPSMA as an alternative to cabazitaxel in men with docetaxel-treated mCRPC.
- Hofman M. 177Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel: Updated results including progression-free survival (PFS) and patient-reported outcomes (PROs) (TheraP ANZUP 1603). Abstract 6, ASCO Genitourinary Cancers Symposium, 11–13 February 2021.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Role of prostate cancer genomics is evolving Next Article
Prognosis of penile cancer associated with HPV status »
« Role of prostate cancer genomics is evolving Next Article
Prognosis of penile cancer associated with HPV status »
Table of Contents: ASCO GU 2021
Featured articles
Prostate Cancer
Lu177 as a promising new therapy for metastatic prostate cancer
Role of prostate cancer genomics is evolving
Apalutamide prolongs progression-free survival in prostate cancer
Dose-intensified radiation therapy fails to provide better outcomes in prostate cancer
Intrinsic tumour biology may be predictive of treatment response in prostate cancer
Final TITAN trial results favour use of apalutamide
Penile Cancer
Prognosis of penile cancer associated with HPV status
Renal Cancer
Superior clinical outcomes and QoL with nivolumab plus cabozantinib in RCC
Lenvatinib plus pembrolizumab prolongs survival in renal cell carcinoma
Inflammatory markers may guide treatment decisions in metastatic renal cell cancer
Clinical trial exclusion criteria may lead to lack of evidence in real-world patients: how do the excluded fare?
Axitinib offers hope for improving renal cell cancer surgical outcomes
Cabozantinib as possible new first-line therapy in translocation renal cell carcinoma
Predictors of oral anti-cancer agent utilisation in renal cell carcinoma
Denosumab plus pembrolizumab in advanced clear cell renal cell carcinoma
Testicular Cancer
New prediction model for brain metastasis in germ cell tumours
Reduction in radiation exposure is possible in testicular seminoma surveillance
New therapeutic option for early metastatic seminoma
Urothelial Cancer
Poorer outcomes in bladder cancer predicted by race/ethnicity and gender
Enfortumab vedotin as a promising treatment option for bladder cancer: phase 3 results
Enfortumab vedotin as a promising treatment option for bladder cancer: phase 2 results
New standard of care recommended for patients with upper tract urothelial cancer
Signature DNA alterations in subtypes of bladder cancer
ACE inhibitors associated with superior responses in bladder cancer
Better allocation of research dollars needed
Better prediction of favourable responses to immune checkpoint inhibitors in mUC
Genitourinary Oncology
Researchers call for an overhaul of licensing and funding of anti-cancer drugs
Exploring a new strategy for metastatic germ cell tumours
Related Articles
February 10, 2021
MRI-targeted biopsy may be of added value in prostate-cancer detection
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

