The SPARTAN trial (NCT01946204) was a multicentre, randomised, double-blinded, phase 3 trial investigating apalutamide for the treatment of nmCRPC [1]. Included men (n=1,207) were randomised 1:2 to receive either apalutamide plus androgen deprivation therapy (ADH; n=401) or placebo plus ADH (n=806) until disease progression, withdrawal of consent, unacceptable toxicity, or death. At the primary endpoint analysis presented at ASCO 2020 and published in the New England Journal of Medicine, both metastasis-free survival and overall survival were significantly improved in the apalutamide arm [2,3].
Prof. Felix Feng (University of California San Francisco, USA) presented results from his team’s analysis of the molecular signatures obtained from the biomarker cohort of the SPARTAN trial (n=233). For this analysis, participants were subdivided into those with early progression (EP) of the disease and those who were long-term responders (LTR). Using archival tissue samples from the biomarker cohort of the SPARTAN trial, researchers aimed to identify molecular signatures associated with either EP or LTR responses to treatment – either by apalutamide (n=60) or placebo (n=37).
Patients with EP or LTR had similar baseline characteristics. Increased immune activity, decreased tumour vascularisation, or decreased proliferative capacity at baseline were associated with LTR in the apalutamide group but not the placebo group (see Table). The inverse was true for EP in the apalutamide group, while increased hormonal independence or metastatic capacity at baseline was associated with EP in the placebo group. Tumours with increased expression of signatures suggestive of T-cell proliferation demonstrated a more favourable response to apalutamide; this response was true in both basal and luminal tumours.
Table: Molecular signatures in the apalutamide + ADT group. Based on [1]
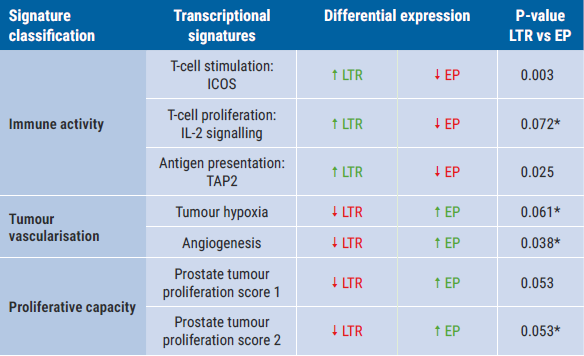
* Reached nominal statistical significance in Cox regression analysis only.
EP, early progression; ICOS, inducible co-stimulatory; IL, interleukin; LTR, long-term responders; TAP2, transporter associated with processing 2.
Prof. Feng concluded that these molecular signatures may be useful to predict which patients with nmCRPC will derive the most favourable treatment responses to apalutamide and other androgen receptor signal inhibitors.
- Feng F. Molecular determinants associated with long-term response to apalutamide in nonmetastatic castration-resistant prostate cancer (nmCRPC). Abstract 8, ASCO Genitourinary Cancers Symposium, 11–13 February 2021.
- Small EJ, et al. Abstract 5516, ASCO Virtual Meeting, 29–31 May 2020.
- Smith MR, et al. N Engl J Med. 2018; 378: 1408–1418.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Final TITAN trial results favour use of apalutamide Next Article
Dose-intensified radiation therapy fails to provide better outcomes in prostate cancer »
« Final TITAN trial results favour use of apalutamide Next Article
Dose-intensified radiation therapy fails to provide better outcomes in prostate cancer »
Table of Contents: ASCO GU 2021
Featured articles
Prostate Cancer
Lu177 as a promising new therapy for metastatic prostate cancer
Role of prostate cancer genomics is evolving
Apalutamide prolongs progression-free survival in prostate cancer
Dose-intensified radiation therapy fails to provide better outcomes in prostate cancer
Intrinsic tumour biology may be predictive of treatment response in prostate cancer
Final TITAN trial results favour use of apalutamide
Penile Cancer
Prognosis of penile cancer associated with HPV status
Renal Cancer
Superior clinical outcomes and QoL with nivolumab plus cabozantinib in RCC
Lenvatinib plus pembrolizumab prolongs survival in renal cell carcinoma
Inflammatory markers may guide treatment decisions in metastatic renal cell cancer
Clinical trial exclusion criteria may lead to lack of evidence in real-world patients: how do the excluded fare?
Axitinib offers hope for improving renal cell cancer surgical outcomes
Cabozantinib as possible new first-line therapy in translocation renal cell carcinoma
Predictors of oral anti-cancer agent utilisation in renal cell carcinoma
Denosumab plus pembrolizumab in advanced clear cell renal cell carcinoma
Testicular Cancer
New prediction model for brain metastasis in germ cell tumours
Reduction in radiation exposure is possible in testicular seminoma surveillance
New therapeutic option for early metastatic seminoma
Urothelial Cancer
Poorer outcomes in bladder cancer predicted by race/ethnicity and gender
Enfortumab vedotin as a promising treatment option for bladder cancer: phase 3 results
Enfortumab vedotin as a promising treatment option for bladder cancer: phase 2 results
New standard of care recommended for patients with upper tract urothelial cancer
Signature DNA alterations in subtypes of bladder cancer
ACE inhibitors associated with superior responses in bladder cancer
Better allocation of research dollars needed
Better prediction of favourable responses to immune checkpoint inhibitors in mUC
Genitourinary Oncology
Researchers call for an overhaul of licensing and funding of anti-cancer drugs
Exploring a new strategy for metastatic germ cell tumours
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

