https://doi.org/10.55788/ca30c32f
Following the pandemic, long COVID or post-acute COVID-19 sequelae have emerged with substantial morbidity. The National Academy of Sciences, Engineering, and Medicine (NASEM) has recently defined long COVID as “an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems” [1].
The cause of long COVID or post-acute sequelae of SARS-CoV-2 (PASC) is most likely multifactorial but may in part be driven by immune dysregulation. Therefore, patients with SARDs are probably at risk for long COVID due to underlying altered immunity. The RheumCARD study was a prospective study of individuals with prevalent SARDs with and without a history of COVID-19. The current analysis by Dr Jeffrey Sparks (Brigham and Women's Hospital, MA, USA) and colleagues aimed to explore whether the presence of circulating inflammatory cytokines after COVID-19 is associated with the occurrence of long COVID in patients with SARDs [2].
The authors measured 48 different circulating cytokines with a panel test (i.e. Olink Target 48 cytokine panel). Participants on DMARDs targeting specific cytokines were excluded. Included were 201 participants with SARD and a history of COVID-19, and 47 participants with SARD but no history of COVID-19. The cohort primarily consisted of women (81%), with a mean age of 56 years. The most common SARDs were inflammatory arthritis (60%) and connective tissue diseases (22%). 39% (79) of the participants with COVID-19 suffered from long COVID (defined in the study as symptoms persisting for ≥28 days) and were analysed for the primary outcome.
Lower IL-18 levels were significantly associated with a diagnosis of long COVID (199 pg/mL vs 221 pg/mL for those without; P=0.001) and a multivariable analysis confirmed this association. Additionally, lower levels of Colony Stimulating Factor (CSF) 2 and the chemokine CCL7, and higher levels of IL-2 were associated with long COVID. The robustness of these findings was confirmed across various subgroups, including those defined by disease activity and SARS-CoV-2 variants.
Thus, lower IL-18 levels were consistently seen in patients with long COVID and SARDs. This finding was robust across analyses and not explained by vaccination or viral variants. IL-18 induces cell-mediated immunity following infection, implicating a blunted immune response as a potential underlying mechanism for long COVID in patients with SARDs. These findings will need replication in the non-SARD population.
- NASEM 2024. Washington, DC: The National Academies Press. DOI: 10.17226/27768.
- Sparks JA, et al. Associations of circulating inflammatory cytokines with long COVID among patients with systemic autoimmune rheumatic diseases. POS0081, EULAR 2024 Congress, 12–15 June, Vienna, Austria.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Stopping DMARDs? These key factors predict RA flares Next Article
Less fracture risk with denosumab than bisphosphonates in pre-treated osteoporosis »
« Stopping DMARDs? These key factors predict RA flares Next Article
Less fracture risk with denosumab than bisphosphonates in pre-treated osteoporosis »
Table of Contents: EULAR 2024
Featured articles
Advanced therapies show promising results in PsA real-world study
Small protein targeting IL-17A effective in PsA management
Late-breaking Abstracts
Nipocalimab meets primary endpoint in Sjögren’s syndrome
Advanced therapies show promising results in PsA real-world study
Dual IL-17A/F blocker significantly reduces spinal radiographic progression in radiographic axSpA
Small protein targeting IL-17A effective in PsA management
Spotlight on Rheumatoid Arthritis
New JAK1 inhibitor outperforms placebo in active RA
Macrophage profiling in synovial tissue predicts treatment response in RA
Innovative app boosts mental health in patients with RA
What is New in Lupus and Scleroderma
Daratumumab shows promise in systemic lupus erythematosus
Severe lung involvement in SSc linked to anti-Ro/SSA antibodies
Early treatment with ambrisentan might prevent PAH development in patients with SSc
Crystal-related Disorders in 2024
More patients hit their uric acid target with febuxostat and ruzinurad combination
Tophaceous gout at higher mortality risk than non-tophaceous gout
JAK Inhibition in Giant Cell Arteritis
Is JAK inhibition the right choice for patients with relapsing giant cell arteritis?
Giant cell arteritis: Upadacitinib may be an upcoming treatment option
Spotlight on Other Indications
AxSpA: Higher comorbidity burden worsens radiographic progression
Hope for a durable effective injection therapy in knee osteoarthritis
Dermatomyositis: Triple therapy with tacrolimus beats cyclosporin regimen
Best of the Posters
Less fracture risk with denosumab than bisphosphonates in pre-treated osteoporosis
Low IL-18: a hidden culprit of long COVID in patients with autoimmune disease
Stopping DMARDs? These key factors predict RA flares
Related Articles
January 18, 2021
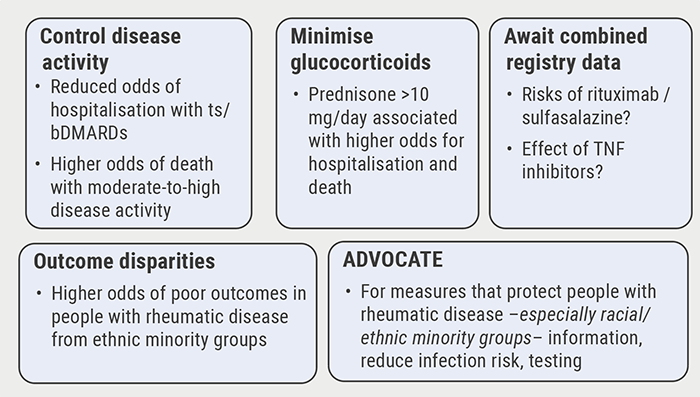
Poor disease control: a risk factor for severe COVID-19
August 14, 2020
New nanoparticle promising future agent in RA
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

