Despite optimised standard-of-care therapy, children with moderate-to-severe asthma may continue to have uncontrolled disease. The IL-4/13 blocker dupilumab has previously been shown to be effective and has a demonstrated favourable safety profile in adolescents and adults with moderate-to-severe asthma [1]. Prof. Leonhard B. Bacharier (Vanderbilt University Medical Center, TN, USA) presented results of the VOYAGE trial (NCT02948959) and pointed out that type 2 inflammation underlies most cases of asthma in children [2]. The VOYAGE trial aimed to assess the efficacy of dupilumab in children aged 6–11 with uncontrolled moderate-to-severe asthma. Enrolled in the trial were 408 children. Patients receiving high-dose inhaled corticosteroids (ICS) alone or a medium-to-high dose ICS with a second asthma controller were randomised 2:1 to receive 100 mg (body weight ≤30 kg) or 200 mg (>30 kg) subcutaneous dupilumab or a matched placebo for up to 52 weeks.
The researchers performed pre-specified primary analyses in 2 populations in the study: 350 patients with markers of type 2 inflammation (baseline blood eosinophils ≥150 cells/μL or fractional exhaled nitric oxide (FeNO) ≥20 ppb) and 259 patients with baseline blood eosinophils ≥300 cells/μL.
At the end of the trial, dupilumab significantly reduced the annualised rate of severe exacerbations versus placebo by 59.3% in the type 2 inflammation phenotype and by 65% in the population with baseline blood eosinophils ≥300 cells/μL (P<0.0001 for both comparisons; see Figure). In addition, the treatment improved the pre-bronchodilator forced expiratory volume in the first second (FEV1) percent of predicted at week 12, a key secondary endpoint, in both populations and reduced FeNO significantly at 12 weeks compared with placebo. The biologic led to rapid and sustained lung function improvement over the entire treatment period in both populations. At week 24, patients treated with dupilumab showed greater improvement in asthma control scores as compared with the placebo group.
Figure: Dupilumab significantly reduced the annualised rate of severe exacerbations versus placebo [2]
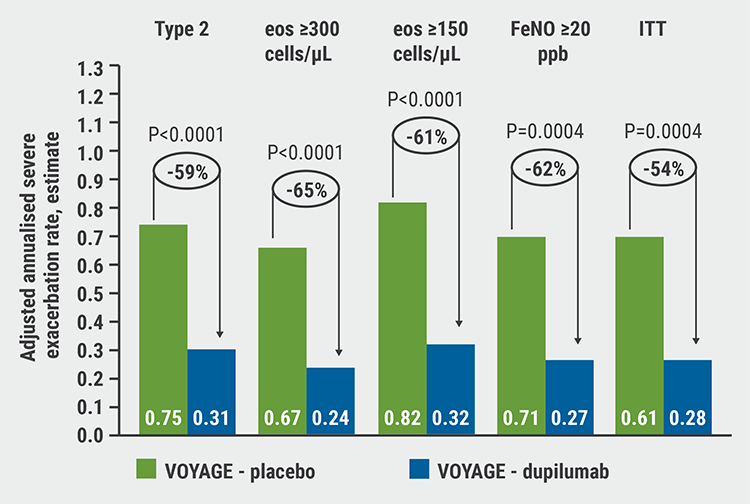
eos, eosinophils; FeNO, fractional exhaled nitric oxide; ITT, intention-to-treat.
The safety profile of dupilumab was generally consistent with the known safety profile of dupilumab in patients aged ≥12 years with moderate-to-severe asthma. “There were 7 parasitic infections, 5 of those were enterobiasis, but they were not serious and did not lead to treatment discontinuation,” Prof. Bacharier explained.
“The effect of dupilumab on improving lung function in these children was particularly impressive,” noted Dr Bacharier. “Decreased lung function is associated with an increased risk of future asthma exacerbations. In addition, impaired lung function may result in abnormal lung growth,” he concluded.
- Castro M, Corren J, Pavord ID, et al. N Engl J Med 2018;378(26):2486-96.
- Bacharier LB, et al. Dupilumab efficacy and safety in children with uncontrolled, moderate-to-severe asthma: The phase 3 VOYAGE study. Session B007: Breaking news: clinical trial results in pulmonary medicine. ATS 2021 International conference, 14-19 May.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Benralizumab lives up to its phase 3 results in real-world findings Next Article
“As-needed” inhaled corticosteroid therapy for mild asthma – what is the evidence? »
« Benralizumab lives up to its phase 3 results in real-world findings Next Article
“As-needed” inhaled corticosteroid therapy for mild asthma – what is the evidence? »
Table of Contents: ATS 2021
Featured articles
Letter from the Editor
COVID-19: What Pulmonologists Need to Know
Antibody treatment for COVID-19: a combination is successful
Air pollution: an underestimated negative prognostic factor for COVID-19
Healthcare workers vulnerable to SARS-CoV-2 infections
Genetic risk variants responsible for COVID-19 predisposition
Asthma – An Update
“As-needed” inhaled corticosteroid therapy for mild asthma – what is the evidence?
IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma
Benralizumab lives up to its phase 3 results in real-world findings
Tezepelumab – good success rates in various types of severe asthma
Sleep Disorders – An Underestimated Problem
OSA: A risk factor for earlier cognitive decline
Subgroup of patients with high heart rate response and coronary artery disease benefit from CPAP
Association between positive airway pressure treatment adherence and COVID-19 infection rates
COPD – What Is New
Possible aetiologies for COPD exacerbations – more evidence is needed
Does COPD plus COVID-19 equal higher mortality?
Biomarkers for acute exacerbations in COPD are required
Severe exacerbations: A key driver of all-cause mortality in COPD patients
Men and women with COPD differ in many ways
Younger adults with COPD at higher health risk than previously thought
Metabolic Dysregulation and Lung Disease
Obesity: A risk factor for new-onset asthma and worse asthma control
Metabolic dysfunction and lung disease: children are no small adults
Best of the Posters
Air pollution in winter linked to more hospital admissions in ILD patients
Tobacco biomarkers do not improve prediction of lung cancer risk
Vaping identified as risk factor for asthma
Related Articles

June 9, 2022
ATS 2022 Highlights Podcast
August 17, 2022
Update on treatment of fibrotic ILD
November 28, 2019
Registry confirms nintedanib efficacy under real-life conditions
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

