After demonstrating significant results in phase 3 trials, the anti-IL-5 antibody benralizumab has been approved by the EMA as add-on maintenance medication in adult patients with severe eosinophilic asthma insufficiently controlled by a combination of inhaled corticosteroids and long-acting beta-agonists [1,2]. This new analysis assessed whether the rates of 51% decreased exacerbations observed in phase 3 would be confirmed in real-world data [3]. The presented retrospective cohort study included patients from medical claims who started benralizumab between 2017 and 2019 [1]. The index date in this pre-post study was the first day after the drug was taken for the first time. Data from 12 months before this day was compared with that of 12 months after the index date. The participants of the primary and secondary cohort were all naïve to biologic treatment and had suffered from ≥2 exacerbations in the pre-index course. The 204 patients of the primary cohort all had ≥2 records of benralizumab, while the secondary or persistent group contained 103 subjects with ≥6 records. Moreover, 114 patients switching from omalizumab and 97 changing from mepolizumab to benralizumab were analysed.
Mean age in the primary cohort was 45.3, 68.6% were women, 77.5% had comorbidity of allergic rhinitis, 45.1% gastroesophageal reflux disease, and 45.6% hypertension. Asthma exacerbation rates were calculated per person-year (PY). The results revealed a 55% reduction (3.25 vs 1.47 per PY) for the benralizumab users within the primary and 62% (3.23 vs 1.23 per PY) in the persistent cohort. Both differences were significant with a P<0.001 for pre- and post-index comparisons. Of note, 41.2% and 42.7% under benralizumab in the primary and persistent cohort, respectively, did not experience any exacerbations during the post-index period. The outcomes for the switch cohorts were similar: 54% reduction when changing from omalizumab and a 34% drop in exacerbations with previous mepolizumab. The ameliorations were also consistent with a decrease in cumulative dosage of oral corticosteroids in the post-index period: in the primary cohort, 82% received steroid maintenance pre-index, but only 50% post-index (P<0.001) (see Figure). “Additionally, rescue medication use, commonly prescribed controllers, and antibiotics also decreased in the post-index period,” Dr Donna Carstens (AstraZeneca, Wilmington, DE, USA) pointed out with regard to significance for short-acting beta-agonists (P<0.001) and the combination of inhaled corticosteroids (ICS), with long-acting β2-adrenergic receptor agonists (P<0.001). In view of the study design that lacked a control arm, further research confirming the results is desirable. “Patients treated with benralizumab in this real-world analysis experienced a significant reduction in asthma exacerbations consistent with pivotal trials, as well as those who switched from other biologics,” concluded Dr Carstens.
Figure: Reduction of oral corticosteroid use among benralizumab receivers [1]
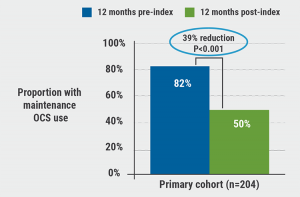
OCS, oral corticosteroids.
- Carstens D, et al. Real-World Effectiveness of Benralizumab on Asthma Exacerbations: Results from the ZEPHYR 1 Study. Session TP015: Updates in Adherence and treatment of lung disease. ATS 2021 International Conference, 14-19 May.
- https://www.ema.europa.eu/en/medicines/human/EPAR/fasenra
- Bleecker ER, et al. Lancet. 2016 Oct 29;388(10056):2115-2127.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Tezepelumab – good success rates in various types of severe asthma Next Article
IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma »
« Tezepelumab – good success rates in various types of severe asthma Next Article
IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma »
Table of Contents: ATS 2021
Featured articles
Letter from the Editor
COVID-19: What Pulmonologists Need to Know
Antibody treatment for COVID-19: a combination is successful
Air pollution: an underestimated negative prognostic factor for COVID-19
Healthcare workers vulnerable to SARS-CoV-2 infections
Genetic risk variants responsible for COVID-19 predisposition
Asthma – An Update
“As-needed” inhaled corticosteroid therapy for mild asthma – what is the evidence?
IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma
Benralizumab lives up to its phase 3 results in real-world findings
Tezepelumab – good success rates in various types of severe asthma
Sleep Disorders – An Underestimated Problem
OSA: A risk factor for earlier cognitive decline
Subgroup of patients with high heart rate response and coronary artery disease benefit from CPAP
Association between positive airway pressure treatment adherence and COVID-19 infection rates
COPD – What Is New
Possible aetiologies for COPD exacerbations – more evidence is needed
Does COPD plus COVID-19 equal higher mortality?
Biomarkers for acute exacerbations in COPD are required
Severe exacerbations: A key driver of all-cause mortality in COPD patients
Men and women with COPD differ in many ways
Younger adults with COPD at higher health risk than previously thought
Metabolic Dysregulation and Lung Disease
Obesity: A risk factor for new-onset asthma and worse asthma control
Metabolic dysfunction and lung disease: children are no small adults
Best of the Posters
Air pollution in winter linked to more hospital admissions in ILD patients
Tobacco biomarkers do not improve prediction of lung cancer risk
Vaping identified as risk factor for asthma
Related Articles
November 28, 2019
Registry confirms nintedanib efficacy under real-life conditions
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

