The traditional management of persistent asthma consists of a controller medication daily and reliever medication as needed. “This procedure was the guideline´s first choice over 3 decades,” Prof. Kaharu Sumino (Washington University School of Medicine, MO, USA) said in her introduction [1]. However, delivery of daily ICS therapy has been challenging, especially due to adherence of <50%. Obviously, enforcing daily ICS when symptoms are not severe is difficult. “Mild asthma can be associated with significant morbidity and mortality,” Prof. Sumino said. A total of 16% of near-fatal asthma and 15% of adults dying from asthma had less than weekly symptoms 3 months before the event [2]. This is the rationale to use non-regular ICS therapies in treating mild asthma, which affects the majority of patients (50–70%) [2]. Potential benefits of as-needed ICS application include a higher sense of self-management by patients. Currently, more evidence is available on as-needed ICS in mild asthma. This reflects in landmark changes in the Global Initiative for Asthma (GINA) guideline in 2019 and the National Asthma Education Prevention Program (NAEPP) guideline in 2020.
Prof. Anne Dixon (University of Vermont, MA, USA) discussed as-needed ICS therapy in adults with mild asthma. In both the GINA and NAEPP guidelines, mild asthma is defined as asthma controlled on step 1 or step 2 treatment, but there are slight differences (see Table) [3]. “What they have in common is the use of as-needed ICS,” Prof. Dixon said. As-needed ICS is what patients do already – they use an inhaler when they have symptoms. Another advantage of providing ICS at the time of symptoms is that anti-inflammatory therapy is given when it is most needed.
Table: Mild asthma treatment options in the NAEPP 2020 update versus GINA 2020 [1]
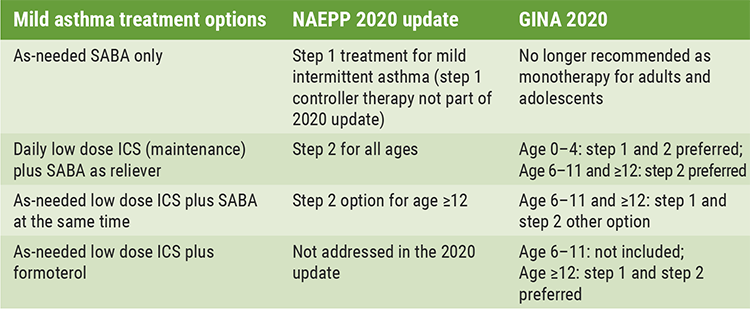
GINA, Global Initiative for Asthma; NAEPP, National Asthma Education Prevention Program; SABA, Short-acting β-agonists; ICS, inhaled corticosteroids.
Only data for the combination budesonide-formoterol
As-needed ICS-formoterol for mild asthma was only studied as budesonide-formoterol combination, a combination that was not yet addressed in the NAEPP update. In the SYGMA 1 trial (NCT02149199) including 3,849 patients with mild asthma that needed step 2 treatment, as-needed budesonide-formoterol provided superior asthma-symptom control to as-needed terbutaline, assessed using electronically recorded weeks with well-controlled asthma for a period of 52 weeks [4]. “Budesonide maintenance was slightly better,” Prof. Dixon said. In the SYGMA 2 trial (NCT02224157), a similar patient population was included (n=4,215) [5]. In this 52-week, non-inferiority trial, budesonide–formoterol used as needed was non-inferior to twice-daily budesonide concerning the rate of severe asthma exacerbations but was inferior in controlling asthma symptoms. Another advantage of the as-needed approach was the median daily dose of ICS being 75% lower in the budesonide–formoterol group. These results are backed by 2 studies reflecting a real-life scenario. In the 52-week, real-world, open-label superiority study PRACTICAL (U1111-1174-2273), 890 patients were included and treated with budesonide–formoterol reliever therapy compared with maintenance budesonide plus as-needed terbutaline. Severe exacerbations per patient per year were lower with as-needed budesonide–formoterol than with maintenance budesonide plus terbutaline as needed. In addition, asthma control did not differ between treatment groups. Again, patients treated with as-needed ICS-formoterol had an ~50% lower ICS dose versus budesonide maintenance [5].
The START trial (2015-002384-42) had a similar result: in this 52-week, real-world, open-label study, 668 asthma patients were either treated with albuterol, budesonide plus as-needed albuterol (budesonide maintenance group) or budesonide-formoterol [6,7]. The annualised exacerbation rate in the budesonide–formoterol group was lower than that in the albuterol group (P<0.001) and did not differ significantly from the rate in the budesonide maintenance group (P=0.65). In both trials, ICS–formoterol-treated patients needed an ~50% reduced ICS dose.
“In conclusion, we have less data regarding as-needed ICS-formoterol for step 1, but very compelling data for step 2,” Prof. Dixon said. As-needed ICS-formoterol reduces the risk of exacerbations compared with as-needed short-acting beta-agonists. In real-world studies, it also reduced severe exacerbations and had a similar effect on symptom scores compared with regularly scheduled ICS, with a lower ICS dose.
- Sumino KC. Introduction of symposium: as-needed ICS therapy in mild asthma, case presentation and guidelines. Session C006: Are we ready for “as-needed” inhaled corticosteroid therapy for mild asthma? Different patients, different perspectives. ATS 2021 International Conference, 14-19 May.
- Dusser D, et al. Allergy 2007;62(6):591-604.
- Dixon A. Adult perspectives of as-needed ICS therapy in mild asthma. Session C006: Are we ready for “as-needed” inhaled corticosteroid therapy for mild asthma? Different patients, different perspectives. ATS 2021 International Conference, 14-19 May.
- O’Byrne PM, et al. N Engl J Med 2018:378(20):1865-76.
- Bateman ED, et al. New Engl J Med 2018;378:1877-87.
- Hardy J, et al. The Lancet 2019;394:919-28.
- Beasley R, et al. New Engl J Med 2019;380:2020-30.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma Next Article
Association between positive airway pressure treatment adherence and COVID-19 infection rates »
« IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma Next Article
Association between positive airway pressure treatment adherence and COVID-19 infection rates »
Table of Contents: ATS 2021
Featured articles
Letter from the Editor
COVID-19: What Pulmonologists Need to Know
Antibody treatment for COVID-19: a combination is successful
Air pollution: an underestimated negative prognostic factor for COVID-19
Healthcare workers vulnerable to SARS-CoV-2 infections
Genetic risk variants responsible for COVID-19 predisposition
Asthma – An Update
“As-needed” inhaled corticosteroid therapy for mild asthma – what is the evidence?
IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma
Benralizumab lives up to its phase 3 results in real-world findings
Tezepelumab – good success rates in various types of severe asthma
Sleep Disorders – An Underestimated Problem
OSA: A risk factor for earlier cognitive decline
Subgroup of patients with high heart rate response and coronary artery disease benefit from CPAP
Association between positive airway pressure treatment adherence and COVID-19 infection rates
COPD – What Is New
Possible aetiologies for COPD exacerbations – more evidence is needed
Does COPD plus COVID-19 equal higher mortality?
Biomarkers for acute exacerbations in COPD are required
Severe exacerbations: A key driver of all-cause mortality in COPD patients
Men and women with COPD differ in many ways
Younger adults with COPD at higher health risk than previously thought
Metabolic Dysregulation and Lung Disease
Obesity: A risk factor for new-onset asthma and worse asthma control
Metabolic dysfunction and lung disease: children are no small adults
Best of the Posters
Air pollution in winter linked to more hospital admissions in ILD patients
Tobacco biomarkers do not improve prediction of lung cancer risk
Vaping identified as risk factor for asthma
Related Articles
November 7, 2018
Gastroesophageal reflux, IPF and lessons learned
July 18, 2022
New guidelines for IPF and PPF
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

