As a high viral load of SARS-CoV-2 was linked to worse outcomes of COVID-19, monoclonal antibodies with the ability to counteract the virus have come into focus for possible treatment [1]. Research results for antibody treatment of other viral infections such as Ebola led Dr Julie V. Philley (University of Texas Health Science Center, USA) and colleagues to hypothesise that a treatment approach with combined antibodies could be beneficial [2]. “The casirivimab and imdevimab combination comprises 2 potent neutralising monoclonal antibodies that bind non-competing epitopes on the SARS-CoV-2 spike protein,“ Dr Philley described in her talk presenting results from the REGN-COV 2067 clinical trial (NCT04425629) [3].
The adaptive phase 1/2/3 study tested dual therapy with casirivimab and imdevimab in outpatients with mild-to-moderate COVID-19. Among the prerequisites for study subjects were central lab-confirmed disease <72 hours and appearance of symptoms ≤7 days prior to randomisation. Initially, randomisation was performed 1:1:1 to 8,000 mg, 2,400 mg, and placebo, but after the analysis of phase 1/2, the protocol was changed with a re-randomisation to 2,400 mg, 1,200 mg, or placebo, eligible only for patients with ≥1 risk factor for severe disease. After a single infusion with casirivimab/imdevimab or placebo on day 1, patients were followed until day 29. The primary endpoint was defined as the proportion of patients with ≥1 hospitalisation or all-cause death, whereas the timespan until COVID-19 symptoms resolved was among the secondary endpoints.
“Patients were well matched across the treatment and the placebo groups: median age was 48.5 years, 58% were obese, 49% were men, and approximately one-third were Hispanic or Latino. Medium viral load was approximately 7 log10 copies and 69% had a negative baseline SARS-CoV-2 serum antibody status,” Dr Philley described the study cohort. She also remarked that the presence of risk factors was well balanced between the study arms.
In the group receiving 2,400 mg of casirivimab/imdevimab, the relative risk of all-cause death decreased by 71% (P<0.0001) (see Figure). Concerning subgroups, COVID-19-related hospitalisations and all-cause death were also significantly reduced. This included patients with high viral loads and high or low baseline seropositivity. The duration of symptoms also decreased by 4 days under casirivimab/imdevimab at both dosages and, again, the result was consistent across the subgroups.
Figure: Comparison of casirivimab/imdevimab versus placebo for all-cause death and COVID-19-induced hospitalisations [3]
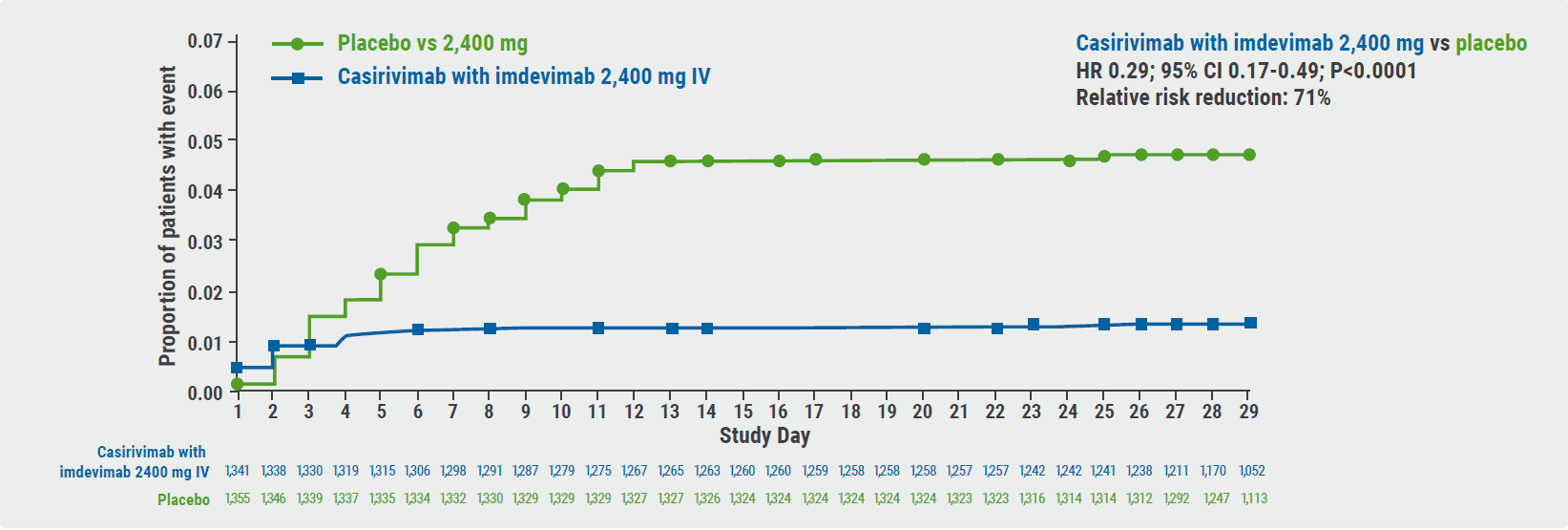
CI, confidence interval; HR, hazard ratio; IV, intravenous; mFAS, modified full analysis set.
In terms of safety, casirivimab/imdevimab was well tolerated with more fatal outcomes in the pooled placebo groups (0.3%) than in the casirivimab/imdevimab arms (0.1% with 1,200 mg; <0.1% with 2,400 mg; and 0% with 8,000 mg).
- Fajnzylber J, et al. Nat Commun. 2020;11(1):5493.
- Mulangu S, et al. N Engl J Med. 2019;381(24):2293-2303.
- Philley JV, et al. Casirivimab with Imdevimab, a cocktail of two antibodies against SARS-CoV-2, in the outpatient setting: phase 3 efficacy and safety results. Session B007: Breaking news: clinical trial results in pulmonary medicine. ATS 2021 International Conference, 14-19 May.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Air pollution: an underestimated negative prognostic factor for COVID-19 Next Article
Tezepelumab – good success rates in various types of severe asthma »
« Air pollution: an underestimated negative prognostic factor for COVID-19 Next Article
Tezepelumab – good success rates in various types of severe asthma »
Table of Contents: ATS 2021
Featured articles
Letter from the Editor
COVID-19: What Pulmonologists Need to Know
Antibody treatment for COVID-19: a combination is successful
Air pollution: an underestimated negative prognostic factor for COVID-19
Healthcare workers vulnerable to SARS-CoV-2 infections
Genetic risk variants responsible for COVID-19 predisposition
Asthma – An Update
“As-needed” inhaled corticosteroid therapy for mild asthma – what is the evidence?
IL-4/13 blocker successful in treatment of paediatric moderate-to-severe asthma
Benralizumab lives up to its phase 3 results in real-world findings
Tezepelumab – good success rates in various types of severe asthma
Sleep Disorders – An Underestimated Problem
OSA: A risk factor for earlier cognitive decline
Subgroup of patients with high heart rate response and coronary artery disease benefit from CPAP
Association between positive airway pressure treatment adherence and COVID-19 infection rates
COPD – What Is New
Possible aetiologies for COPD exacerbations – more evidence is needed
Does COPD plus COVID-19 equal higher mortality?
Biomarkers for acute exacerbations in COPD are required
Severe exacerbations: A key driver of all-cause mortality in COPD patients
Men and women with COPD differ in many ways
Younger adults with COPD at higher health risk than previously thought
Metabolic Dysregulation and Lung Disease
Obesity: A risk factor for new-onset asthma and worse asthma control
Metabolic dysfunction and lung disease: children are no small adults
Best of the Posters
Air pollution in winter linked to more hospital admissions in ILD patients
Tobacco biomarkers do not improve prediction of lung cancer risk
Vaping identified as risk factor for asthma
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

