"This study demonstrates that early response assessed by PET to dual HER2-blockade with trastuzumab and pertuzumab could identify those patients who are more likely to achieve a pathological complete response (pCR)," said lead study author Dr. Jose Manuel Perez-Garcia of the International Breast Cancer Center, Quiron Group, in Barcelona, Spain.
"This is the first study conducted in patients with HER2-positive early breast cancer that has an adaptive design and treats a group of patients (those included in arm B with PET response and a pCR) exclusively with trastuzumab and pertuzumab without chemotherapy," he told Reuters Health by email.
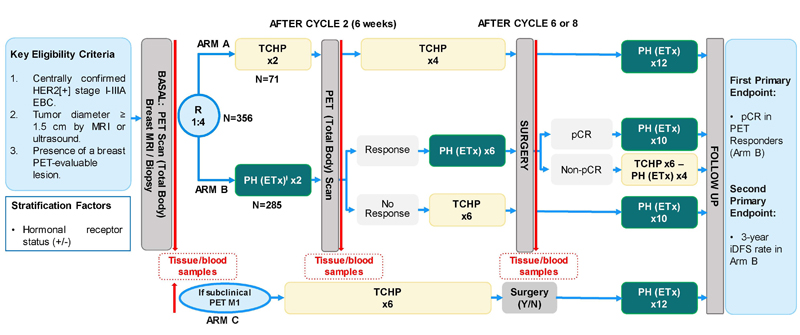
As reported in The Lancet Oncology, the researchers studied adult women with HER2-positive, stage-I-IIIA, invasive, operable breast cancer. Eligible participants had at least one breast lesion evaluable by F18-FDG-PET, Eastern Cooperative Oncology Group performance status of 0 or 1, and baseline left ventricular ejection fraction of 55% or higher.
Over a two-year period, patients were stratified by hormone-receptor status and randomly assigned to one of two treatment groups at 45 hospitals in Europe.
One group, with 71 patients, received docetaxel (75 mg/m2), carboplatin (area under the concentration- time curve, 6 mg/mL/min), trastuzumab (600 mg fixed dose), and pertuzumab (840 mg loading dose, 420 mg maintenance doses).
The other group, with 285 patients, received trastuzumab and pertuzumab. Hormone-receptor-positive patients in this second group also received tamoxifen if premenopausal (20 mg/day orally) or letrozole if postmenopausal (2.5 mg/day orally). Participants underwent F18-FDG-PET scans before they were randomized and after two treatment cycles.
All patients in the first group completed six cycles of treatment every three weeks, while everyone treated without chemotherapy initially received two cycles of trastuzumab and pertuzumab. PET responders the second group continued the treatment for six more cycles, while PET non-responders in this group were switched to six cycles of docetaxel, carboplatin, trastuzumab, and pertuzumab.
Surgery was performed within six weeks after the last treatment dose.
With a median follow-up was 5.7 months, 80% of patients treated without chemotherapy were PET responders and 38% of these had a pathological complete response, significantly more than the historical rate. Serious adverse events included neutropenia and febrile neutropenia, but no deaths occurred during neoadjuvant treatment.
By comparison, 86% of the patients on chemotherapy were PET responders and 66% of these had a pathological complete response.
"The results are not surprising," Dr. Perez-Garcia said, "because prior clinical evidence had already shown a significant rate of pCR with exclusive dual HER2-blockade."
"The main strength of the study is its strategic and adaptive design, while the only weakness could be the number of patients included in the study," he added.
Senior author Dr. Javier Cortes, also of the International Breast Cancer Center, told Reuters Health by email, "PET imaging is generally available, and these findings represent an important step forward in the management of this patient population, reducing side effects and offering a clear improvement in quality of life."
But he cautioned, "Before recommending this de-escalation strategy, it is critical that we wait for the results of the second co-primary endpoint of the study, the 3-year invasive disease-free survival."
F Hoffmann-La Roche funded the study and provided trastuzumab and pertuzumab. Drs. Perez-Garcia and Cortes and several of their coauthors report financial ties to the company.
SOURCE: https://bit.ly/3c8UJ5i The Lancet Oncology, online May 18, 2021.
By Lorraine L. Janeczko
Posted on
Previous Article
« Vertex cystic fibrosis treatment gets U.S. approval for use in 6-11 year olds Next Article
Lung-cancer screening can spot people at high risk for aortic stenosis »
« Vertex cystic fibrosis treatment gets U.S. approval for use in 6-11 year olds Next Article
Lung-cancer screening can spot people at high risk for aortic stenosis »
Related Articles
January 11, 2024
monarchE: No predictive biomarkers revealed with molecular profiling
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

