https://doi.org/10.55788/354082eb
Dr Kohei Shitara (National Cancer Center Hospital East, Japan) presented the third scheduled interim analysis of the phase 3 KEYNOTE-585 trial (NCT03221426), which assessed the efficacy of peri-operative pembrolizumab combined with chemotherapy versus placebo with chemotherapy in patients with untreated, locally advanced, resectable gastric or gastro-oesophageal junction (G/GEJ) cancer [1]. The initial results did not show a significant improvement in event-free survival (EFS), though the pCR rate did improve [2].
Participants (n=804) were randomised 1:1 to receive neoadjuvant pembrolizumab (200 mg IV every 3 weeks) or a placebo, combined with chemotherapy (cisplatin plus capecitabine or cisplatin plus 5-FU) for 3 cycles [1]. Post-surgery, participants received adjuvant pembrolizumab or placebo plus chemotherapy every 3 weeks for 3 cycles, followed by adjuvant pembrolizumab or placebo every 3 weeks for 11 cycles. In a separate FLOT cohort, participants were randomised to receive either pembrolizumab or placebo with docetaxel, oxaliplatin, leucovorin, and 5-FU every 2 weeks. The primary endpoints were pCR, EFS, OS, and safety in the FLOT cohort.
With a median follow-up of 59.9 months, the pCR rate was 13.4% with pembrolizumab plus chemotherapy versus 2.0% with placebo plus chemotherapy, showing a difference of 11.4%. The median EFS was 44.4 months with pembrolizumab plus chemotherapy versus 25.5 months with placebo plus chemotherapy (HR 0.81; 95% CI 0.67–0.99); median OS was 71.8 versus 55.7 months, respectively (HR 0.86; 95% CI 0.71–1.06). Grade ≥3 drug-related adverse event rates were 65% in the pembrolizumab group versus 63% in the placebo group.
The efficacy and safety outcomes were thus consistent with previous analyses, although the pCR in the control arm is low compared with that in the original FLOT4 trial [3]. Improvement in EFS was not statistically significant, raising questions about the overall benefit of adding pembrolizumab to the treatment regimen [1]. The discussant, Dr Lizzy Smyth (Oxford University Hospitals NHS Foundation Trust, UK) pointed out that immunologically “hot” tumours, namely those that have high microsatellite instability or have PD-L1 expression >10%, showed the greatest pCR improvement (38% increase; see Figure) as well as marked improvements in EFS and OS (both with HR 0.60). This subgroup of patients would likely benefit in the short- and long-term from peri-operative pembrolizumab.
Figure: Efficacy outcomes of KEYNOTE-585 in key biomarker groups [1]
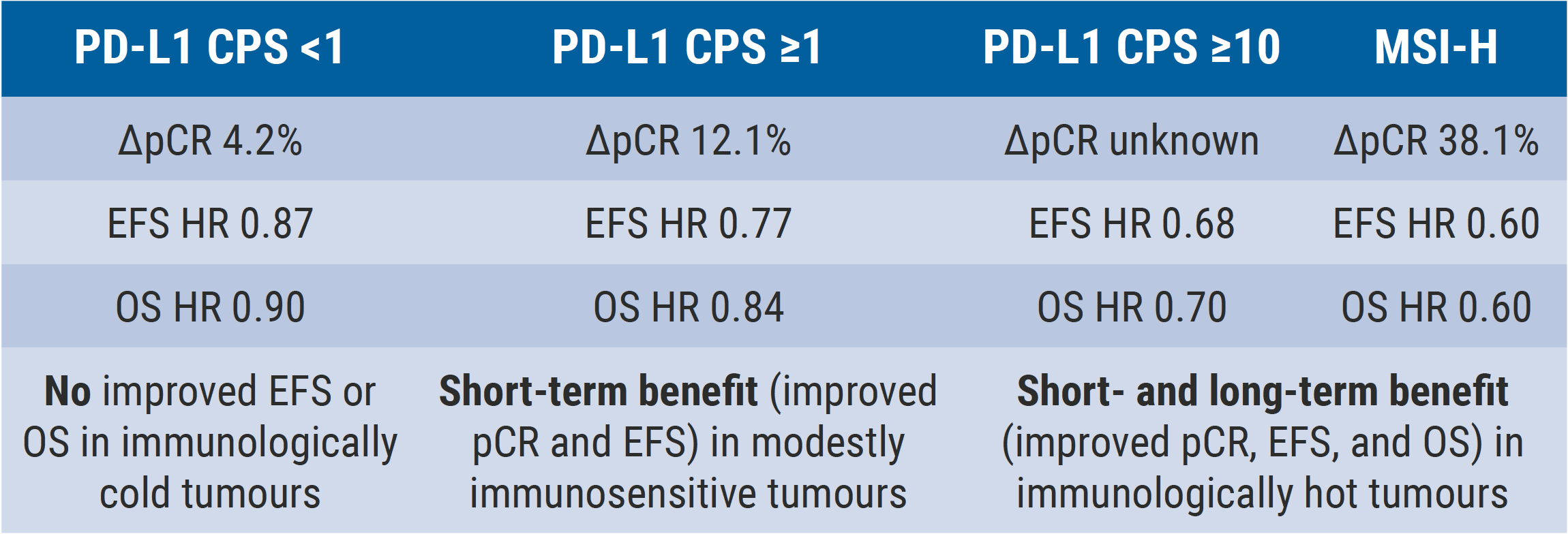
CPS, combined positive score; EFS, event-free survival; MSI-H, microsatellite instability-high; OS, overall survival; pCR, pathological complete response.
- Shitara K, et al. Final analysis of the phase 3 KEYNOTE-585 study of pembrolizumab plus chemotherapy vs chemotherapy as perioperative therapy in locally-advanced gastric and gastroesophageal junction cancer. Abstract LBA3, ESMO Gastrointestinal Cancers Congress 2024, 26–29 June, Munich, Germany.
- Shitara K, et al. Lancet Oncol. 2024;25(2):212-224.
- Al-Batran SE, et al. 2019 May 11;393(10184):1948-1957.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Prognostic value of ctDNA in stage III colon cancer Next Article
High efficacy of pembrolizumab combined with standard therapy in patients with MSS/pMMR mCRC and high immune infiltrate »
« Prognostic value of ctDNA in stage III colon cancer Next Article
High efficacy of pembrolizumab combined with standard therapy in patients with MSS/pMMR mCRC and high immune infiltrate »
Table of Contents: ESMO GI 2024
Featured articles
Gastric and Oesophageal Cancer
OS benefit in ARMANI, but is it worth it?
SPOTLIGHT on new targets in immunotherapy: claudin 18.2
Encouraging efficacy of anti-claudin 18.2 ADC in G/GEJ cancer
New analyses validate TAP and CPS scores for PD-L1 expression
KEYNOTE-585: negative trial, but long-term benefit in PD-L1-high/MSI subgroups?
Cancers of the Pancreas, Small Bowel, and Hepatobiliary Tract
AI facilitates early detection of hepatocellular carcinoma
177Lu-DOTATATE significantly extends PFS in patients with GEP-NETs, regardless of grade or origin
Durvalumab plus chemotherapy enhances 3-year survival in advanced biliary tract cancer
Promising first results of mitazalimab in metastatic pancreatic ductal adenocarcinoma
Cancers of the Colon, Rectum, and Anus
Post-operative MRD status more prognostic than TNM stage
CAPRI 2 GOIM trial navigates biomarker-driven therapy
Meta-analysis of triplet therapy in BRAFV600E-mutated mCRC
CheckMate 8HW: Nivolumab/ipilimumab in MSI-H/dMMR mCRC
Sequence effect for third-line treatment of mCRC
REGINA meets stage 1 endpoint in rectal cancer and moves to stage 2 with reduced dose regorafenib
High efficacy of pembrolizumab combined with standard therapy in patients with MSS/pMMR mCRC and high immune infiltrate
Prognostic value of ctDNA in stage III colon cancer
Neoadjuvant combined immunotherapy also effective in MSS/pMRR CRC
General GI Cancer
Peri- or post-operative chemotherapy benefits patients with resectable CRCLM
MINOTAUR: Promising phase 1 data for lunresertib plus FOLFIRI
Related Articles
October 23, 2019
IBD prevalence 3 times higher than estimated and expected to rise
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

