https://doi.org/10.55788/e9df06fa
There are currently no standard first-line treatment options for patients with higher grade 2–3, well-differentiated, advanced GEP-NETs. Recently, results from the phase 3 NETTER-2 trial (NCT03972488) showed that 177Lu-DOTATATE plus octreotide (30 mg) significantly improved progression-free survival (PFS) compared with high dose octreotide (60 mg) in patients with somatostatin receptor-positive, higher grade GEP-NETs [1]. Now, Dr Simron Singh (University of Toronto, Canada) presented results from a pre-planned subgroup analysis of the trial [2].
NETTER-2 randomised 226 participants to first-line treatment with 177Lu-DOTATATE plus octreotide (n=151) or high dose octreotide alone (control arm, n=75). In the total study population, the median PFS was 22.8 months in the 177Lu-DOTATATE arm versus 8.5 months in the control arm (HR 0.276; 95% CI 0.182–0.418; P<0.0001) [1].
The subgroup analysis demonstrated a benefit of 177Lu-DOTATATE both in participants with grade 2 (n=142) or grade 3 (n=79) GEP-NETs: median PFS of 29.0 versus 13.8 months (HR 0.306) and of 22.0 versus 5.6 months (HR 0.266), respectively [2]. In addition, a benefit of 177Lu-DOTATATE was observed regardless of the primary tumour origin (see Figure). The median PFS in participants with pancreatic NETs (n=123) was 19.4 versus 8.5 months (HR 0.336); in participants with small intestine NETs (n=66) it was 29.0 versus 8.4 months (HR 0.305). Median PFS in participants with other GEP-NETs (n=37) was not shown.
Figure: PFS benefit with 177Lu-DOTATATE for patients with GEP-NETs [2]
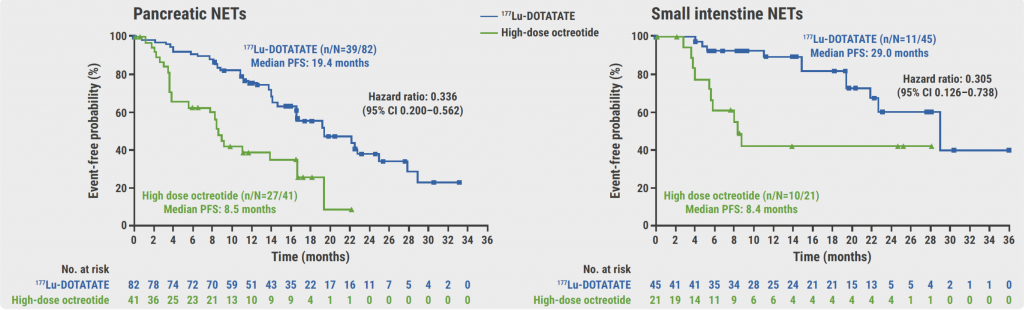
GEP-NETs, gastroenteropancreatic neuroendocrine tumours; PFS, progression-free survival.
Objective response rates for 177Lu-DOTATATE were 40.4%, 48.1%, 51.2%, and 26.7% in participants with grade 2, grade 3, pancreatic, and small intestine NETs, respectively. The median duration of response was 24.9 months, 19.3 months, 18.4 months, and not yet reached in the respective subgroups.
Based on these results, Dr Singh concluded that “first-line 177Lu-DOTATATE plus octreotide should be considered a standard-of-care for patients with advanced, well-differentiated, grade 2 or 3, somatostatin receptor-positive GEP-NETs.”
- Singh S, et al. Lancet. 2024;403(10446):2807-2817.
- Singh S, et al. First-line efficacy of [177Lu]Lu-DOTA-TATE in patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors by tumor grade and primary origin: subgroup analysis of the phase 3 NETTER-2 study. Abstract 211MO, ESMO Gastrointestinal Cancers Congress 2024, 26–29 June, Munich, Germany.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Durvalumab plus chemotherapy enhances 3-year survival in advanced biliary tract cancer Next Article
Encouraging efficacy of anti-claudin 18.2 ADC in G/GEJ cancer »
« Durvalumab plus chemotherapy enhances 3-year survival in advanced biliary tract cancer Next Article
Encouraging efficacy of anti-claudin 18.2 ADC in G/GEJ cancer »
Related Articles

March 12, 2021
Borderline resectable pancreatic cancer: phase 2 results
March 21, 2022
Persisting disparities in pancreatic cancer care
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

