ATTRACTION-3 is a phase 3, multicentre, randomised, open-label global study, although 96% of patients in both treatment arms were from Asia. Patients were treated until disease progression or unacceptable toxicity. A total of 419 patients were enrolled: 210 were allocated to nivolumab and 209 to chemotherapy.
For the primary endpoint of overall survival (OS), nivolumab demonstrated a statistically significant improvement over chemotherapy (see Table), with a 23% reduction in risk of death (HR 0.77; 95% CI 0.62-0.96; P=0.019) and a 2.5-month improvement in median OS (10.9 months; 95% CI 9.2-13.3) compared with patients treated with chemotherapy (8.4 months; 95% CI 7.2-9.9). The safety profile of nivolumab in this trial was consistent with previously reported studies in ESCC and other solid tumours.
Table. Results from the ATTRACTION-3 trial, which demonstrated superior OS and a favourable safety profile vs CT in pts with previously treated advanced ESCC, with survival benefit observed regardless of tumour PD-L1 expression
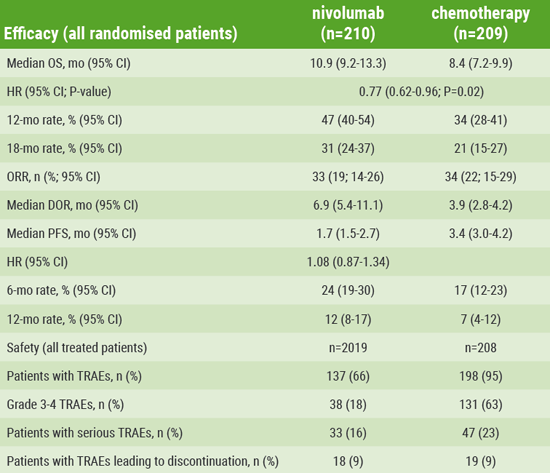
Table provided by ESMO
Patients treated in the nivolumab arm showed 12- and 18-month OS rates of 47% (95% CI 40-54) and 31% (95% CI 24-37), respectively, vs 34% (95% CI 28-41) and 21% (95% CI 15-27) among patients in the chemotherapy arm. Survival benefit with nivolumab was observed regardless of tumour PD-L1 expression levels. An exploratory analysis of patient-reported outcomes showed significant overall improvement in quality of life with nivolumab vs chemotherapy.
The objective response rates between the two arms were comparable at 19% (95% CI 14-26) among patients receiving nivolumab vs 22% (95% CI 15-29) among those receiving chemotherapy. However, the study showed nivolumab substantially increased the median duration of response for patients (6.9 months; 95% CI 5.4-11.1) vs 3.9 months (95% CI 2.8-4.2). In total, 7 patients in the nivolumab arm had ongoing responses at data cut-off compared with 2 patients in the chemotherapy arm. An overall HR of 1.08 (95% CI 0.87-1.34) suggested no meaningful difference in progression-free survival between the nivolumab and chemotherapy arms.
Fewer treatment-related adverse events (AEs) were reported with nivolumab vs chemotherapy, with a rate of 66% of any grade treatment-related AEs for patients receiving nivolumab compared with 95% for patients receiving chemotherapy. Patients in the nivolumab arm also experienced a lower incidence of grade 3 or 4 treatment-related AEs compared with those in the chemotherapy arm (18% vs 63%), and the percentage of patients experiencing treatment-related AEs leading to discontinuation was the same in both arms (9%).
“The significant survival benefit coupled with the favourable safety profile and patient-reported outcomes observed in this trial suggest nivolumab has the potential to represent an important new second-line treatment option for patients with advanced oesophageal squamous cell carcinoma, offering the possibility to extend their survival and improve their quality of life during treatment,” said Prof. Cho.
- Cho BC et al. ESMO Congress 2019. Abstract LBA 11.
- Kato K et al. Lancet Oncol. 2019 Sep 27. pii: S1470-2045(19)30626-6.
Posted on
Previous Article
« Adjuvant nivolumab provides benefit Next Article
Enfortumab vedotin and pembrolizumab in advanced bladder cancer: initial results »
« Adjuvant nivolumab provides benefit Next Article
Enfortumab vedotin and pembrolizumab in advanced bladder cancer: initial results »
Table of Contents: ESMO 2019
Featured articles
Interview with ESMO President Prof. Josep Tabernero
Breast Cancer
Triple negative breast cancer gets positive news: KEYNOTE-522 interim results
CDK4/6 inhibitors change landscape of breast cancer treatment: 2 studies
Veliparib-chemo combo prolongs survival without disease progression in some advanced breast cancer patients
Lung Cancer
Improved response rates without survival benefit with pembrolizumab in pretreated mesothelioma
Frontline ipilimumab/nivolumab improves OS in advanced NCLSC
First-line osimertinib significantly lengthens OS in NSCLC
Liquid biopsy to decide the best treatment for NSCLC
Melanoma
Long-term data from CheckMate 067
Adjuvant nivolumab provides benefit
Nivolumab+ipilimumab superior to monotherapy for melanoma brain metastases
GI Cancers
Preoperative chemotherapy for colon cancer
Nivolumab improves OS in advanced oesophageal cancer
Liquid biopsy identifies relapse in patients with colorectal cancer after surgery
In hepatocellular carcinoma, CheckMate 459 misses OS endpoint, but some interesting trends emerge
Heavily pre-treated GIST: ripretinib improves PFS
FGFR2+ cholangiocarcinoma: pemigatinib active as second-line treatment
IDH1+ cholangiocarcinoma: phase 3 results show improved PFS
Advanced colorectal cancer and BRAF mutations: triplet combination improves survival
Genitourinary Cancers
25% reduction in the risk of death in patients with nmCRPC treated with apalutamide
Enfortumab vedotin and pembrolizumab in advanced bladder cancer: initial results
PARP inhibition in selected patients slows progression on advanced prostate cancer
PFS extension with immunotherapy + chemotherapy in urothelial cancer
Third-line in mCRPC: CARD trial
Prostate cancer: spare radiotherapy after surgery
Novel mode of action for kidney cancer treatment
Gynaecological Cancers
Ovarian cancer patients benefit from combined maintenance therapy
Combination of PARP inhibition plus chemotherapy in ovarian cancer
PFS benefit with niraparib as first-line maintenance in ovarian cancer
CNS Tumours
Ceritinib in ALK+ NSCLC brain metastases
Solid Tumours/Pan-Tumour Data
Mixed data: AMG 510 in tumours with KRASG12C
DNA profiling of carcinoma of unknown primary should inform treatment
Larotrectinib: safe and effective in TRK fusion-positive tumours
Related Articles
November 26, 2019
Combination of PARP inhibition plus chemotherapy in ovarian cancer

November 26, 2019
Mixed data: AMG 510 in tumours with KRASG12C
November 26, 2019
IDH1+ cholangiocarcinoma: phase 3 results show improved PFS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

