Patients with TNBC who have residual invasive disease after completion of neoadjuvant chemotherapy have a very high risk of recurrence [1]. Recently, the CREATE-X trial showed this risk is reduced by adjuvant capecitabine, while pre-clinical models suggest TNBC basal subtype has increased sensitivity for platinum-based chemotherapy [2,3].
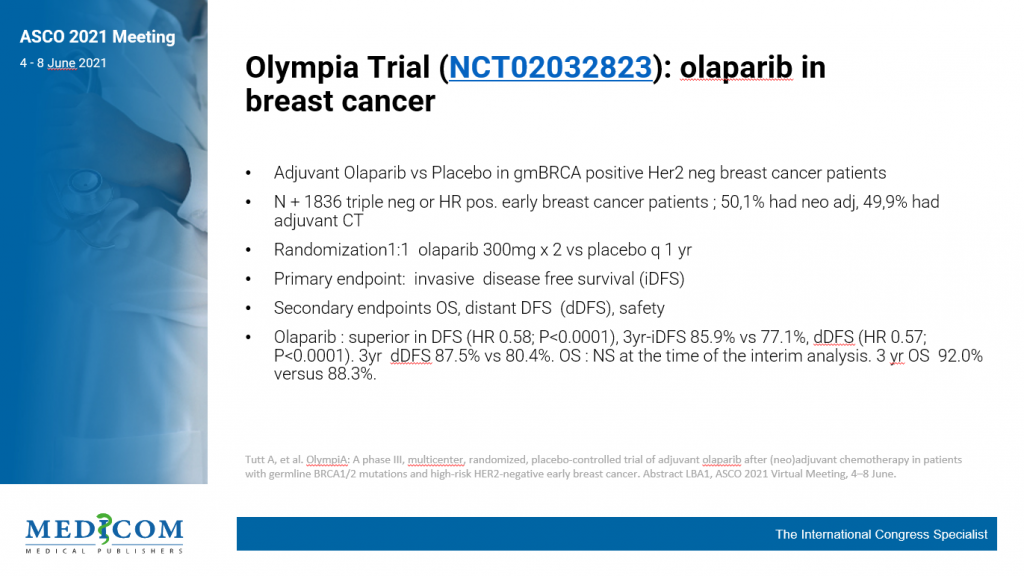
Therefore, the randomised, phase 3 ECOG-ACRIN EA1131 trial (NCT0244539) tested the hypothesis that invasive disease-free survival would be improved in patients with basal subtype TNBC with residual disease after neoadjuvant chemotherapy with the adjuvant use of platinum-based chemotherapy instead of capecitabine. The trial enrolled 410 patients (recruitment goal: 775) with clinical stage II/III TNBC post-neoadjuvant taxane ± anthracycline-based chemotherapy with at least 1 cm residual disease in the surgical specimen. Patients were randomised 1:1 to receive platinum-based adjuvant chemotherapy (carboplatin or cisplatin once every 3 weeks for 4 cycles) or capecitabine (14 out of 21 days every 3 weeks for 6 cycles). TNBC subtype (i.e basal and non-basal) was analysed in the surgical specimen by PAM50 profiling. The primary endpoint of the trial was invasive disease-free survival. A non-inferiority design (non-inferiority margin of HR of 1.154) with superiority alternative (alternative HR of 0.754) was chosen, assuming a 4-year invasive disease-free survival of 67% for the capecitabine arm. Prof. Ingrid Mayer (Vanderbilt University School of Medicine, TN, USA) presented the results of ECOG-ACRIN EA1131 after the fifth interim analysis [4].
After a median follow-up of 20 months and 120 invasive disease-free survival events (61% of full information) in the 308 (78%) patients with basal subtype TNBC, the 3-year invasive disease-free survival for platinum-treated patients was 42% versus 49% for capecitabine-treated patients (HR 1.06; 95% CI 0.62-1.81).
Grade 3 and 4 toxicities were more common with platinum agents. Based on these interim results, the Data and Safety Monitoring Committee recommended stopping the trial as it was unlikely that further follow-up would show non-inferiority or superiority of platinum-based chemotherapy.
- Symmans WF, et al. J Clin Oncol. 2017;35:1049-1060.
- Masuda N, et al. N Engl J Med. 2017;376:2147-2159.
- Lehmann BD, et al. J Clin Invest. 2011;121:2750-2767.
- Mayer IA, et al. A randomized phase III post-operative trial of platinum-based chemotherapy (P) versus capecitabine (C) in patients (pts) with residual triple-negative breast cancer (TNBC) following neoadjuvant chemotherapy (NAC): ECOG-ACRIN EA1131. Abstract 605, ASCO 2021 Virtual Meeting, 4–8 June.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Dual HER2-blockade shows anti-tumour activity in patients with uterine cancer Next Article
Sustained efficacy of nivolumab/ipilimumab plus 2 cycles of chemotherapy in NSCLC »
« Dual HER2-blockade shows anti-tumour activity in patients with uterine cancer Next Article
Sustained efficacy of nivolumab/ipilimumab plus 2 cycles of chemotherapy in NSCLC »
Table of Contents: ASCO 2021
Featured articles
Downloadable 1-Page Editor-Selected Trial PowerPoint Slides
Breast Cancer
Excellent prognosis for breast cancer patients with ultra-low-risk gene signature
Olaparib benefits early breast cancer patients with BRCA1/2 germline mutation
Platinum-based adjuvant chemotherapy in TNBC is not superior or non-inferior to capecitabine
Dalpiciclib benefits patients with HR-positive, HER2-negative advanced breast cancer
Trastuzumab-deruxtecan showed clinical activity in patients with brain metastases
Lung Cancer
Neoadjuvant nivolumab plus chemotherapy improves surgical outcomes in NSCLC
Immune-related adverse events are associated with efficacy of atezolizumab in patients with advanced NSCLC
Sustained efficacy of nivolumab/ipilimumab plus 2 cycles of chemotherapy in NSCLC
Patritumab deruxtecan (HER3-DXd) in EGFR TKI-resistant NSCLC
Melanoma
Long-term results from ground-breaking melanoma trials
Novel dual checkpoint blockade improves progression-free survival in melanoma
Neoadjuvant therapy with nivolumab plus relatlimab is safe and effective in patients with stage III melanoma
Genitourinary Cancers
VISION trial shows improved survival with 177Lu-PSMA-617 in mCRPC
Abiraterone added to ADT + docetaxel nearly doubles survival in de novo mCSPC
Post-nephrectomy pembrolizumab improves disease-free survival
Glutaminase inhibitor telaglenastat does not improve survival mRCC
Promising efficacy and safety of feladilimab in recurrent/metastatic urothelial carcinoma
Gastrointestinal Cancers
Pembrolizumab benefits survival in MSI-H/dMMR metastastic colorectal cancer
Panitumumab added to 5-FU/LV effective as maintenance therapy in patients with mCRC
Trastuzumab-deruxtecan showed promising activity in patients with HER2-expressing mCRC
Benefit of both I-O/chemo combo and I-O/I-O combo over chemotherapy alone in oesophageal squamous cell cancer
Benefit of I-O/chemo combo over chemotherapy alone in advanced GC/GEJC/EAC
Perioperative chemotherapy and neoadjuvant multimodality therapy appear equally effective
Haematological Cancers
Olutasidenib demonstrates efficacy in patients with relapsed/refractory IDH1 mutant AML
Acalabrutinib as effective but better tolerated than ibrutinib in CLL
Gynaecological Cancers
Adjuvant chemotherapy does not improve outcome in patients with locally advanced cervical cancer
Novel drug combination for recurrent ovarian cancer
Dual HER2-blockade shows anti-tumour activity in patients with uterine cancer
Paediatric Cancer
Molecular tumour profiling impacts the diagnosis and treatment of solid tumours
Circulating tumour DNA to evaluate response in children with neuroblastoma
Basic Science
PARP7 inhibitor shows promising results in first-in-human trial
IACS-6274 is well tolerated and biologically active in selected advanced tumours
CYT-0851 shows promising anti-tumour activity across different tumour types
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com